|
METALLURGICAL AND MATERIALS TRANSACTIONS B
|

|
ABSTRACTS
Volume 26B, No. 3, June 1995
This Month Featuring: The 1994 Distinguished Lecture in Materials and Society, The 1993 Edward DeMille Campbell Memorial Lecture, The 1993 Distinguished Lecture in Materials and Society, Mineral Preparation, Hydrometallurgy, Pyrometallurgy, Electrometallurgy, Transport Phenomena, Physical Chemistry, Solid State Reactions, Surface Treatment, Mathematical Modeling. View June 1995 Table of Contents.
|
THE 1994 DISTINGUISHED LECTURE IN MATERIALS AND SOCIETY
A 50 Year View of Materials Science: 30 Down and 20 to Go
PETER R. BRIDENBAUGH
THE 1993 EDWARD DEMILLE CAMPBELL MEMORIAL LECTURE
Concrete: The Advanced Industrial Material of the 21st Century
CAROLYN M. HANSSON
Portland cement concrete is presented as the most widely used, yet most ignored--by the materials science and engineering community--advanced industrial material. The complexity and the time, temperature, and humidity dependence of its structure and properties are described. Concrete can be produced anywhere in the world at reasonable strengths, but concrete structures are failing, not because of lack of intrinsic strength, but largely because of their exposure to increasingly hostile environments. Current research efforts in understanding the structure-property relationships and in increasing the durability of concrete are reviewed.
THE 1993 DISTINGUISHED LECTURE IN MATERIALS AND SOCIETY
Materials Technology and the Materials Industry: A Critical Transition
DONALD R. MUZYKA
World events are rapidly restructuring our materials industry, Transitions in research and development, education, the organization of corporations, and world economics affect our personal and professional lives. Evidence of change and the forces that produce it are omnipresent. The strategies for the materials industry to prosper in the new world order are not obvious. The sources of funding that have been the traditional basis for the advancement of materials are now in question. Small independent companies, which once had active materials development programs, find it difficult to undertake such efforts in the present economic climate. The way in which corporations and countries compete in the world is the foremost challenge that our industry faces in the 1990s. Partnerships between U.S. industry and national laboratories are finally becoming a reality and have the potential to make progress in our industry more rapid. Even partnerships between U.S. companies and institutions in the former Soviet Union are being realized! If we are able to restructure the way we fund and do research, the opportunities to advance materials science and engineering are endless. On the horizon is the Engineered Materials Age, in which we will be able to put together, materials that are lighter, stronger, more conductive, more resilient, and smarter and some that have "memories."
MINERAL PREPARATION
Kinetics of Roasting of Chromium Oxide with Sodium Nitrate Flux
A.K. TRIPATHY, H.S. RAY, and P.K. PATTNAYAK
Low temperature isothermal kinetic studies were performed on alkali roasting of Cr203 with progressive replacement of Na2CO3 in the reaction mixture by NaNO3 in the temperature range of 573 to 873 K. The influence of the nitrate on the course of the reactions was studied. Statistical design of experiments was employed to assess the relative influence of different process variables. Kinetic analysis of the experimental data shows that the reaction with NaNO3 follows a simple first-order model, and the activation energy of the reaction is 22.7 kJmol-1, as compared to 72 kJmol-1 for Na2CO3 flux.
HYDROMETALLURGY
The Bioleaching of Different Sulfide Concentrates Using Thermophilic Bacteria
F. TORRES, M.L. BLÁZQUEZ, F. GONZÁLEZ, A. BALLESTER, and J.L. MIER
The bioleaching of different mineral sulfide concentrates with thermophilic bacteria (genus Sulfolobus) was studied. Since the use of this type of bacteria in Ieaching systems involves stirring and the control of temperature, the influence of the type of stirring and the pulp density on dissolution rates was studied in order to ascertain the optimum conditions for metal recovery. At low pulp densities, the dissolution kinetic was favored by pneumatic stirring, but for higher pulp densities, orbital stirring produced the best results. A comparative study of three differential concentrates, one mixed concentrate, and one global concentrate was made. Copper and iron extraction is directly influenced by bacterial activity, while zinc dissolution is basically due to an indirect mechanism that is activated in the presence of copper ions. Galvanic interactions between the different sulfides favors the selective bioleaching of some phases (sphalerite and chalcopyrite) and leads to high metal recovery rates. However, the formation of galvanic couples depends on the type of concentrate.
PYROMETALLURGY
Simultaneous Optimization of Thermochemical Data for Liquid Iron Alloys Containing C, N, Ti, Si, Mn, S, and P
DOMINIQUE BOUCHARD and CHRISTOPHER W. BALE
A data base of thermochemical parameters for liquid iron-base alloys containing C, N, Ti, Si, Mn, S, and P is presented. A matrix of linear inequalities describing the experimental data among these elements was constructed. A set of internally consistent thermochemical data permitted by the uncertainties of the experiments was evaluated by means of a linear programming algorithm. Expressions for the interaction parameters of the solutes and the Gibbs energies of formation of the carbides, nitrides, carbonitrides, and sulfides of titanium were simultaneously optimized. It is shown that the resulting thermochemical data base reproduces the experimental data satisfactorily.
Simultaneous Reduction and Carburization of Ilmenite
K.S. COLEY, B.S. TERRY, and P. GRIEVESON
Western Australian ilmenite was reduced using "Collie" coal at temperatures in the range 1587 to 1790 K to form carbon-saturated iron and titanium oxycarbide. The oxycarbide phase formed from Ti3O5 at temperatures below 1686 K and from Ti2O3 at temperatures above 1686 K. At 1686 K both mechanisms occurred. The reaction rate was controlled by oxidation of carbon by carbon dioxide generated by reduction of the oxide phase. The final product at temperatures up to 1686 K was a fine dispersion of titanium oxycarbide in iron. At 1790 K, the reducing oxide tended to remain intact and formed a coarser distribution. In general, manganese impurities from the ilmenite were confined to the iron phase in the product, although some of the coarser oxycarbide particles formed at 1790 K contained trapped manganese at the internal pores.
Derivation and Consistency of the Partial Functions of the Ternary System Involving Interaction Coefficients
J.P. HAJRA, S. RAVINDRA REDDY, and M.G. FROHBERG
The logarithm of activity coefficients of the components of the ternary system is derived based on the Maclaurin infinite series, which is expressed in terms of the integral property of the system and subjected to appropriate boundary conditions. The derivation of the functions involves extensive summation of various infinite series pertaining to the first-order interaction coefficients that have been shown completely to remove any truncational error. Since the conventional equations involving interaction coefficients are internally inconsistent, a consistent form of the partial functions is developed in the article using the technique just described. The thermodynamic consistency of the functions based on the Maxwell and the Gibbs-Duhem relations has been established. The derived values of the logarithmic activity coefficients of the components have been found to be in agreement with the thermodynamic data of the Fe-Cr-Ni system at 1873 K and have been found to be independent of the compositional paths.
Constant-Pressure Specific Heat to Hemispherical Total Emissivity Ratio for Undercooled Liquid Nickel, Zirconium, and Silicon
AARON J. RULISON and WON-KYU RHIM
Radiative cooling curves of nickel, zirconium, and silicon melts that were obtained using the high-temperature, high-vacuum electrostatic levitator (HTHVESL) have been analyzed to determine the ratio between the constant-pressure specific heat and the hemispherical total emissivity, cp(T)/ T(T). This ratio determined over a wide liquid temperature range for each material allows us to determine cp(T) if
T(T). This ratio determined over a wide liquid temperature range for each material allows us to determine cp(T) if  T(T) is known or vice versa. Following the recipe, the hemispherical total emissivities for each sample at its melting temperature,
T(T) is known or vice versa. Following the recipe, the hemispherical total emissivities for each sample at its melting temperature,  T(Tm), have been determined using cp(Tm) values available in the literature. They are 0.15,0.29, and 0.17, for Ni, Zr, and Si, respectively.
T(Tm), have been determined using cp(Tm) values available in the literature. They are 0.15,0.29, and 0.17, for Ni, Zr, and Si, respectively.
Thermoanalysis of the Combined Fe3O4-Reduction and CH4-Reforming Processes
A. STEINFELD, A. FREI, and P. KUHN
The chemical equilibrium composition of the system Fe3O4 + 4CH4 at 1300 K and 1 atm consists of solid Fe and a 2:1 gas mixture of H2 and CO. Thermogravimetric (TG) analysis combined with gas chromatographic measurements was conducted on the reduction of Fe3O4 (powder, 2-µm mean particle size) with 2.3, 5, 10, and 20 pct CH4 in Ar, at 1273, 1373, 1473, and 1573 K. The reduction proceeded in two stages, from Fe3O4 to FeO, and finally to Fe. CH4 conversion and H2 yield increased with temperature, while the overall reaction rate increased with temperature and CH4 concentration. C (gr) deposition, due to the cracking of CH4, was observed. By applying a topochemical model for spherical particles of unchanging size, the reaction mechanism was found to be mostly controlled by gas boundary layer diffusion. The apparent activation energy reached a maximum at 30 pct reduction extent and decreased monotonically until completion. When compared with the results using instead either H2 or CO as reducing gas, the reduction achieved completion faster using CH4 at temperatures above 1373 K.
Oxidation of Cobaltite: Part I. Process Mineralogy
G.X. WANG, D. CHANDRA, and M.C. FUERSTENAU
The control of arsenic is important in oxidation roasting and leaching of cobaltite. Oxidation roasting below 823 K did not show any crystal structural change in cobaltite, but heating to 923 K yielded Co-As-oxide, whose composition is varied. Direct oxidation of cobaltite to cobalt oxides with traces of arsenic was observed by roasting at 1023 K or above. The distribution of arsenic within the particles gradually decreased from the outer region to the center of the particles. The arsenic concentration within the particles decreased with the increase of temperature, suggesting temperature dependence on arsenic removal from the lattice of cobaltite. In this article, the process mineralogy of the oxidation of cobaltite is presented at various temperatures. The results from X-ray diffraction (XRD) analysis, reflected light microscopic analysis, and scanning electron microscopic analysis of the roasted cobaltite concentrates together with a rationalized thermodynamic analysis are presented.
Oxidation of Cobaltite: Part II. Kinetics
G.X. WANG, D CHANDRA, and M.C. FUERSTENAU
A kinetics study has been performed on cobaltite to understand the oxidation processes over a temperature range of 573 to 1173 K using a thermogravimetric method. The results show that oxidation of cobaltite occurs in two stages. In the first stage which occurs between 823 and 913 K, the majority of the sulfur is removed. However, the arsenic remains in the lattice of the reacted region. A pore-blocking kinetic model yields a satisfactory fit to these experimental data. At higher temperatures, there is a concurrent release of As and S from the crystal lattice of CoAsS. The shrinking-core kinetic model is applicable. Complementary X-ray diffraction and scanning electron microscopic analyses on these partially oxidized samples support the kinetic models. The effects of partial pressure of oxygen and particle size on roasting have been evaluated.
Communication: A Study on Thermal Oxidation of Burnt Zirconium Fines in a Fluidized-Bed Reactor
S.P. CHAKRABORTY, I.G. SHARMA, and D.K. BOSE
ELECTROMETALLURGY
Extension of the k- Model for the Numerical Simulation of the Melt Flow in Induction Crucible Furnaces
Model for the Numerical Simulation of the Melt Flow in Induction Crucible Furnaces
E. BAAKE, A. MÜHLBAUER, A. JAKOWITSCH, and W. ANDREE
Checking the calculations of turbulent melt flow in induction crucible furnaces that were carried out with various modifications of the two-dimensional (2-D) k- model using experimental findings has shown basic differences in the distributions of the specific generation of the turbulent energy and the kinetic energy of the turbulence. The discrepancies are explained by the distinctive three-dimensional (3-D) character of the pulsations and the low-frequency fluctuations of the macroscopic toroidal eddy; these are not taken into account in the numerical methods mentioned. With the aid of this 3-D model, the additional component of turbulent kinetic energy involved is estimated, and an approximation formula for the low-frequency component of the specific generation of turbulence is given. This results in an extension of the 2-D k-
model using experimental findings has shown basic differences in the distributions of the specific generation of the turbulent energy and the kinetic energy of the turbulence. The discrepancies are explained by the distinctive three-dimensional (3-D) character of the pulsations and the low-frequency fluctuations of the macroscopic toroidal eddy; these are not taken into account in the numerical methods mentioned. With the aid of this 3-D model, the additional component of turbulent kinetic energy involved is estimated, and an approximation formula for the low-frequency component of the specific generation of turbulence is given. This results in an extension of the 2-D k- model for a recirculated flow with several toroidal eddies, leading to good qualitative agreement of the characteristics of turbulent flow with the experimental findings. Since the numerical simulation--in agreement with industrial practice and the experiments carried out--demonstrates good effective mixing of the entire flow region, there is thus a possibility for the simulation of material transport in the melt of induction crucible furnaces as part of the widespread 2-D computation methods.
model for a recirculated flow with several toroidal eddies, leading to good qualitative agreement of the characteristics of turbulent flow with the experimental findings. Since the numerical simulation--in agreement with industrial practice and the experiments carried out--demonstrates good effective mixing of the entire flow region, there is thus a possibility for the simulation of material transport in the melt of induction crucible furnaces as part of the widespread 2-D computation methods.
TRANSPORT PHENOMENA
Direct Simulation of Initial Filtration Phenomena within Highly Porous Media
CHENGUO TIAN and R.I.L. GUTHRIE
Initial filtration phenomena within highly porous filter media--ceramic foam filters (CFF)--were simulated by numerically solving the Navier-Stokes equations and the general transport equation for suspended particles. In this approach a "piece" of the highly porous filter was modeled directly by constructing a calculation domain such that the main average geometrical properties of the real filters were embodied therein. The governing equations were then solved by imposing appropriate boundary conditions on the solid surfaces of the filter webs. The influences of Reynolds, Peclet, and Gravitational numbers as well as filter porosity on initial filtration efficiencies were investigated in this simulation. Predicted results were spot-checked against data from industrial filtration trials conducted by the authors for aluminum melts and found to be in good agreement.
Communication: Grain Agglomeration in Solid-Liquid Mixtures under Microgravity Conditions
RANDALL M. GERMAN
PHYSICAL CHEMISTRY
Kinetic Studies of Reduction of CoO and CoWO4 by Hydrogen
J.A. BUSTNES, DU SICHEN, and S. SEETHARAMAN
The present work deals with the studies of the kinetics of reduction of CoO and CoWO4 in flowing hydrogen gas by thermogravimetric method. The reduction studies for CoO were carried out in the temperature range of 637 to 837 K, while for CoWO4 reduction, the temperature range was 837 to 1173 K. In the case of the reduction of CoWO4, the reaction products after reduction were analyzed by X-ray diffraction as well as scanning electron microscopy. The activation energy for the reduction of CoO was found to be of 54.3 kJ/mol. Cobalt tungstate was reduced to a mixture of Co7W6 and W, and the activation energy for the reaction is 90.0 kJ/mol. The results arc discussed in the light of the reduction kinetics of other transition metal oxides and tungstates under similar conditions.
Thermodynamics of Na2O in the Molten CaO-CaF2-SiO2 System
HISAO KIMURA, FUMITAKA TSUKIHASHI, and NOBUO SANO
The activity of a few percent of Na2O in the CaO-CaF2-SiO2 system doubly saturated with CaO and 3CaO · SiO2 has been determined between 1423 and 1623 K by a chemical equilibration technique. The temperature dependence of the activity coefficient of Na2O may be expressed as follows.
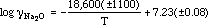
It was found that Henry's law holds up to 5 mass pct of Na2O in the flux at 1473 K. The activity of a small amount of Na2O in the CaO-CaF2-SiO2 flux is discussed in comparison with that for the Na2O-SiO2 system in terms of their refining characteristics.
Determination of the Standard Gibbs Energy for the Reaction of 3BaO (s) + 2Cr (s) + 3/2O2 (g) = 3BaO · Cr2O3 (s)
YOSHINAO KOBAYASHI, KAZUKI MORITA, and NOBUO SANO
The standard Gibbs energy formation of 3BaO · Cr2O3 has been measured by a chemical equilibrium technique and is expressed as follows:
3BaO (s) + 2Cr (s) +  O2 (g) = 3BaO · Cr2O3 (s)
O2 (g) = 3BaO · Cr2O3 (s)
 G° = -1,260,000 (±3000) + 282 (±24)T(J/mol)
G° = -1,260,000 (±3000) + 282 (±24)T(J/mol)
The activity coefficient of chromium in copper, which was needed for the foregoing measurement, may be expressed by the following equation:
log 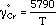 - 2.10
- 2.10
The value of standard Gibbs energy at 1573 K was found to be close to that of ereaction of formation of CaO · Cr2O3 expressed in the similar form. The BaO saturated BaF2 flux is shown to be far more promising in the oxidative dephosphorization of chromium-containing hot metal in comparison with the CaO-SiO2-CaF2 flux doubly saturated with CaO and 3CaO · Cr2O3.
Thermodynamics of Inclusion Formation in Fe-Cr-Ti-N Alloys
BAHRI OZTURK, R. MATWAY, and R.J. FRUEHAN
The thermodynamics of titanium in Fe-Cr alloys and of inclusion formation in Fe-Cr-N-Ti alloys was investigated. A metal-nitride-gas equilibration technique was used to measure the activity of titanium. The equilibrium titanium content of the metal that is in equilibrium with pure solid titanium nitride and nitrogen gas at 1 atm was determined. The activity coefficients of titanium (fTi) relative to 1 wt pct standard state in Fe were calculated for Fe-Cr alloys from the experimental results. The first-order interaction coefficient between titanium and chromium,  , was determined to be 0.024 at 1873 K. The solubility of nitrogen in Fe-Cr alloys was measured and was found to increase with chromium content, which is in agreement with previous work. Thermodynamic calculations were made in order to predict under what conditions titanium nitride will form in 409 stainless steel and was compared with inclusions found in plant samples. The inclusion stability diagrams for 304 stainless steel and Fe-18 pct Cr and Fe-9 pct Cr alloys were computed.
, was determined to be 0.024 at 1873 K. The solubility of nitrogen in Fe-Cr alloys was measured and was found to increase with chromium content, which is in agreement with previous work. Thermodynamic calculations were made in order to predict under what conditions titanium nitride will form in 409 stainless steel and was compared with inclusions found in plant samples. The inclusion stability diagrams for 304 stainless steel and Fe-18 pct Cr and Fe-9 pct Cr alloys were computed.
On the Thermodynamic Behavior of Cadmium in Te-Saturated HgTe-CdTe and CdSe-CdTe Solid Alloys .
M. SHAMSUDDIN and A. NASAR
The activity of cadmium in Te-saturated HgTe-CdTe and CdSe-CdTe alloys in the temperature range of 720 to 840 K was measured by an electrochemical technique using LiCl-KCl + 5 wt pct CdCl2 as the molten salt electrolyte. From the electromotive force (emf), values measured at different temperatures, the partial and excess molar thermodynamic quantities, viz.,  ,
, 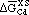 ,
,  ,
, 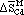 , and
, and 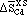 , have been calculated. From the activity data, the equilibrium vapor pressure of cadmium over the alloys have been estimated. The thermodynamic data obtained in the present investigation are consistent with the view that the HgTe-CdTe system consists of a single-phase field throughout the entire range of composition and with the structural changes in the CdSe-CdTe system. The results have been discussed in the light of Darken's stability and excess stability parameters of the systems and physical properties, viz., energy gap and magnetic susceptibility.
, have been calculated. From the activity data, the equilibrium vapor pressure of cadmium over the alloys have been estimated. The thermodynamic data obtained in the present investigation are consistent with the view that the HgTe-CdTe system consists of a single-phase field throughout the entire range of composition and with the structural changes in the CdSe-CdTe system. The results have been discussed in the light of Darken's stability and excess stability parameters of the systems and physical properties, viz., energy gap and magnetic susceptibility.
A Rigorous Equation of State for Solids, Liquids, and Gases
G.W. TOOP
In this research report, a rigorous equation of state is derived. Only thermodynamic relationships are used in the derivation, and hence, no restrictive or conditional model is assumed. The advantage of an equation of slate that is based on thermodynamic principles is that, by definition, it is applicable to all substances, solid, liquid, or gas. It can, therefore, be used to provide a theoretical insight into other equations of state, notably the van der Waals equation, and as a consistency check for measured thermodynamic data in general. The behavior of the terms in the equation, at and near the critical point, is defined.
Communication: A New Method for Measuring Activities in Slags Containing a Volatile Component
CHANGHONG DAI, XIANPENG ZHANG, and LI SHUI
Communication: Solubilities of Molybdenum in Liquid Tin
RYO KAWABATA, MUNETAKA MYOCHIN, and MASANORI IWASE
Commmunication: Discussion of "Thermodynamic Stability of Metallurgical Coke Relative to Graphite"
B.S. TERRY and X. YU
Communication: Authors' Reply
K.T. JACOB and S. SEETHARAMAN
SOLID STATE REACTIONS
The Effect of an Electric Field on Self-Sustaining Combustion Synthesis: Part I. Modeling Studies
A. FENG and Z.A. MUNIR
An analysis of the effect of an electric field on self-propagating high-temperature synthesis (SHS) reactions is presented. Using the synthesis of SiC as a model, the analysis showed that the imposition of a field results in a highly localized distribution of the current density. It was shown that the current is primarily restricted to the region just ahead of the combustion zone. Thus, in addition to the chemical heat release, this zone also includes heat release from an electric source, a value equivalent to  E2 where
E2 where  is the conductivity and E is the field. From the dependence of the degree of conversion to the product on the applied voltage, it is shown that the velocity of the combustion wave is linearly proportional to the field.
is the conductivity and E is the field. From the dependence of the degree of conversion to the product on the applied voltage, it is shown that the velocity of the combustion wave is linearly proportional to the field.
The Effect of an Electric Field on Self-Sustaining Combustion Synthesis: Part II. Field-Assisted Synthesis of  -SiC
-SiC
A. FENG and Z.A. MUNIR
Self-sustaining combustion synthesis of  -SiC is shown to be possible in the presence of an electric field. Above a threshold field of ~6.8 V cm-1, a combustion wave resulting from the reaction between silicon and graphite powders can be self-sustaining. A linear relationship between the applied field and the measured wave velocity is observed, in qualitative agreement with the model (as discussed in the previous article). At relatively high fields, >21 V · cm-1, simultaneous combustion occurs. Microstructural examinations of quenched combustion fronts provided evidence of melting of the Si reactants. This experimental work provides evidence of the validity of a model in which chemical and electrical heat generation are contributing inside the combustion front.
-SiC is shown to be possible in the presence of an electric field. Above a threshold field of ~6.8 V cm-1, a combustion wave resulting from the reaction between silicon and graphite powders can be self-sustaining. A linear relationship between the applied field and the measured wave velocity is observed, in qualitative agreement with the model (as discussed in the previous article). At relatively high fields, >21 V · cm-1, simultaneous combustion occurs. Microstructural examinations of quenched combustion fronts provided evidence of melting of the Si reactants. This experimental work provides evidence of the validity of a model in which chemical and electrical heat generation are contributing inside the combustion front.
Kinetic Analysis of the Combustion Synthesis of Molybdenum and Titanium Silicides
LILY L. WANG and Z.A. MUNIR
The temperature profiles associated with the passage of self-propagating combustion waves during the synthesis of MoSi2 and Ti5Si3 were determined. From these profiles, kinetic analyses of the combustion synthesis process for these two silicides were made. The synthesis is associated with high heating rates: 1.3 x 104 and 4.9 x 104 K · s-1 for MoSi2 and Ti5Si3, respectively. The width of the combustion zone was determined as 1.3 and 1.8 mm for the silicides of Mo and Ti, respectively. The degree of conversion,  , and its spatial distribution and the conversion rate,
, and its spatial distribution and the conversion rate, 
 /
/  t, were determined. However, because of the inherent characteristics of wave propagation in MoSi2, only in the case of Ti5Si3, could the activation energy be calculated. An average value of 190 · kJ mol-1 was determined for titanium silicide.
t, were determined. However, because of the inherent characteristics of wave propagation in MoSi2, only in the case of Ti5Si3, could the activation energy be calculated. An average value of 190 · kJ mol-1 was determined for titanium silicide.
The Synthesis of Nickel Aluminides by Multilayer Self-Propagating Combustion
T.S. DYER and Z.A. MUNIR
The synthesis of Ni-Al intermetallic thin films by self-propagating combustion reactions was investigated for the 1:1 and 3:1 Ni/Al stoichiometries. The dependence of the combustion wave velocity on the individual layer thickness was determined. The marked decrease in velocity with layer thickness was consistent with results of modeling studies on multilayer systems. Activation energies for the synthesis of NiAl were determined to be in the range 127.9 to 149.8 kJ · mol-1, and those for the synthesis of Ni3Al were found to be in the range 133.8 to 146.3 kJ · mol-1. In the case of NiAl, the experimental value is attributed to a diffusion process of Al in NiAl. Differential thermal analysis (DTA) showed the sequence of steps in the formation of NiAl and Ni3Al. The dependence of the thermal peaks on the heating rate for both cases was found to be consistent with theory. The activation energies obtained from the DTA analysis were compared to previous results obtained with relatively thin layers.
Communication: Gibbs Free Energy of Ca3Si2O7--A Reassessment of Electromotive Force Measurements
K.T. JACOB
SURFACE TREATMENT
Communication: Determination of the Absorptivity of Al-33Cu Wt Pct Alloy during Laser Treatment by an Inverse Method
A. ZRYD, M. GREMAUD, and A. MATSUNAWA
MATHEMATICAL MODELING
Theoretical Investigation of Penetration Characteristics in Gas Metal-Arc Welding Using Finite Element Method
SUBODH KUMAR and S.C. BHADURI
In the modeling of the gas metal-arc (GMA) welding process, heat inputs to the workpiece by the arc and the metal transfers have been considered separately. The heat energy delivered due to the metal transfer has been approximated in the form of a cylindrical volumetric heat source, whose dimensions of the radius and the height are dependent on the molten metal droplet characteristics. The pinch instability theory (PIT) and the static force balance theory (SFBT) of drop detachment have independently been used to obtain the expressions for various characteristics of the drop, i.e., the drop radius, the drop velocity, and the drop frequency at various welding parameters. The occurrence or the nonoccurrence of finger penetration, routinely found in the GMA welding at high welding currents, has been satisfactorily explained by the cylindrical heat source model. The effect of various welding parameters, e.g., the welding current, the wire radius etc., on the weld bead penetration characteristics has been investigated. In this modeling effort, the heat conduction equation has been solved in three dimensions.
The Impact of Bubble Dynamics on the Flow in Plumes of Ladle Water Models
Y.Y. SHENG and G.A. IRONS
Bubbly plumes are widely encountered in metallurgical processes when gas is injected into liquid metals for refining purposes. Based on the experimental findings from a water model ladle, this phenomenon was simulated with a mathematical model paying special attention to the dynamics of the bubbles in the plume In the model, the liquid flow field is first calculated in an Eulerian frame with an estimated distribution of the void fraction The trajectories of bubbles are then computed in a Lagrangian manner using the estimated flow field, experimentally measured information on bubble drag coefficients, lateral migration due to lateral lift forces, and variation in bubble size due to breakup. Turbulence in the two-phase zone is modeled with a modified k- model with extra source terms to account for the second phase. The computed void fraction and turbulent liquid flow field distributions are in good agreement with experimental measurements.
model with extra source terms to account for the second phase. The computed void fraction and turbulent liquid flow field distributions are in good agreement with experimental measurements.
Experimental and Theoretical Study of Particle Dispersion Phenomena in a Turbulent Gas Jet of the Flash-Smelting Process by the Image Analysis Technique
YUTAKA YASUDA and H.Y. SOHN
The particle dispersion phenomena in a turbulent gas jet of the flash-smelting process have been investigated. The particle number density in a nonreacting jet was measured experimentally by taking photographs of the particles in flight and counting them using the image analysis technique. Numerical computations were also performed using a mathematical model for a turbulent particle-laden gas jet. The experimental particle number density data were then compared with the predicted results of the model. Good agreement was obtained between the experimental and predicted data. The effects of the parameters such as the injector design, particle loading, and air flow rate are discussed. Optimization of the concentrate burner design can be achieved with the help of experimental and theoretical simulations as performed in this work.
Correction
Contents:
Metallurgical and Materials Transactions A, Volume 26A, May 1995
Contents:
Metallurgical and Materials Transactions A, Volume 26A, June 1995
Direct questions about this or any other Metallurgical and Materials Transactions page to mettrans@andrew.cmu.edu.

 T(T). This ratio determined over a wide liquid temperature range for each material allows us to determine cp(T) if
T(T). This ratio determined over a wide liquid temperature range for each material allows us to determine cp(T) if  Model for the Numerical Simulation of the Melt Flow in Induction Crucible Furnaces
Model for the Numerical Simulation of the Melt Flow in Induction Crucible Furnaces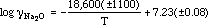
 O2 (g) = 3BaO · Cr2O3 (s)
O2 (g) = 3BaO · Cr2O3 (s) G° = -1,260,000 (±3000) + 282 (±24)T(J/mol)
G° = -1,260,000 (±3000) + 282 (±24)T(J/mol)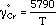 - 2.10
- 2.10 , was determined to be 0.024 at 1873 K. The solubility of nitrogen in Fe-Cr alloys was measured and was found to increase with chromium content, which is in agreement with previous work. Thermodynamic calculations were made in order to predict under what conditions titanium nitride will form in 409 stainless steel and was compared with inclusions found in plant samples. The inclusion stability diagrams for 304 stainless steel and Fe-18 pct Cr and Fe-9 pct Cr alloys were computed.
, was determined to be 0.024 at 1873 K. The solubility of nitrogen in Fe-Cr alloys was measured and was found to increase with chromium content, which is in agreement with previous work. Thermodynamic calculations were made in order to predict under what conditions titanium nitride will form in 409 stainless steel and was compared with inclusions found in plant samples. The inclusion stability diagrams for 304 stainless steel and Fe-18 pct Cr and Fe-9 pct Cr alloys were computed.
 ,
, 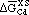 ,
,  ,
, 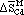 , and
, and 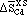 , have been calculated. From the activity data, the equilibrium vapor pressure of cadmium over the alloys have been estimated. The thermodynamic data obtained in the present investigation are consistent with the view that the HgTe-CdTe system consists of a single-phase field throughout the entire range of composition and with the structural changes in the CdSe-CdTe system. The results have been discussed in the light of Darken's stability and excess stability parameters of the systems and physical properties, viz., energy gap and magnetic susceptibility.
, have been calculated. From the activity data, the equilibrium vapor pressure of cadmium over the alloys have been estimated. The thermodynamic data obtained in the present investigation are consistent with the view that the HgTe-CdTe system consists of a single-phase field throughout the entire range of composition and with the structural changes in the CdSe-CdTe system. The results have been discussed in the light of Darken's stability and excess stability parameters of the systems and physical properties, viz., energy gap and magnetic susceptibility.
 E2 where
E2 where  -SiC
-SiC -SiC is shown to be possible in the presence of an electric field. Above a threshold field of ~6.8 V cm-1, a combustion wave resulting from the reaction between silicon and graphite powders can be self-sustaining. A linear relationship between the applied field and the measured wave velocity is observed, in qualitative agreement with the model (as discussed in the previous article). At relatively high fields, >21 V · cm-1, simultaneous combustion occurs. Microstructural examinations of quenched combustion fronts provided evidence of melting of the Si reactants. This experimental work provides evidence of the validity of a model in which chemical and electrical heat generation are contributing inside the combustion front.
-SiC is shown to be possible in the presence of an electric field. Above a threshold field of ~6.8 V cm-1, a combustion wave resulting from the reaction between silicon and graphite powders can be self-sustaining. A linear relationship between the applied field and the measured wave velocity is observed, in qualitative agreement with the model (as discussed in the previous article). At relatively high fields, >21 V · cm-1, simultaneous combustion occurs. Microstructural examinations of quenched combustion fronts provided evidence of melting of the Si reactants. This experimental work provides evidence of the validity of a model in which chemical and electrical heat generation are contributing inside the combustion front.
 , and its spatial distribution and the conversion rate,
, and its spatial distribution and the conversion rate, 