Common fuels—whether they are
simple hydrocarbons or oxygenated
hydrocarbons or carbohydrates—can
be economically produced from the respective
oxides of carbon and hydrogen
by simple chemical reductions. These
reactions are efficiently carried out by
applying fundamentals of the reduction
process.
INTRODUCTION
…describe the overall significance
of this paper?
CO2 reduction in the atmosphere can
be carried out by chemical reduction
of CO2 and/or H2O into fuels (by
applying extractive metallurgy knowhow
for reducing oxides) as well as
reduction of usage, as discussed in
the papers forming the symposium.
…describe this work to a materials
science and engineering professional
with no experience in your
technical specialty?
Extractive metallurgists know how to
reduce oxide ores into metals. This
knowledge can be used to reduce
oxides of carbon and hydrogen
into non-global-warming matter.
This approach is as beneficial as
minimizing reducing agent carbon
and hydrogen consumption.
…describe this work to a layperson?
Some metal ores are converted to
metals using energy other than
carbon-based fuels—such as
electricity, CO2, and H2O—which
are similar to metal ores and can
be modified to non-global-warming
forms by known techniques by
extractive metallurgists.
|
When converting minerals into metals,
energy is consumed. Even though
energy in various forms can be used
in achieving this conversion, or reduction,
typically a reductant that can carry
the anion of the mineral away from the
desired element is used. The potential
for economic production of common
fuels from the respective oxides of carbon
and hydrogen by simple chemical
reductions1 was the inspiration for the
CO2 Reduction Metallurgy Symposium
at the TMS 2008 Annual Meeting. The
TMS Reactive Metals Committee initiated
the symposium, as carbon’s reactivity
is well known to all.
The world needs fuels in all three
states of matter: solid, liquid, and gaseous.
Naturally available solar, wind,
and hydro-energy can be converted into
the mobile forms of fuel by using carbon
dioxide and water. When such conversions
can be affordably achieved,
perhaps one cause of global warming—
carbon emissions—will be minimized.
THERMAL EMISSIONS
Most of the gaseous emissions from
the use of fuels are emitted at temperatures
higher than ambient temperature.
This mass of gases, when mixed with
ambient atmosphere, increases the atmospheric
air mass temperature. Present-day fuels release both CO2 and H2O,
along with hot air, in their exhaust. Of
these, the non-condensible CO2 (under
atmospheric conditions) continues to
increase and is easily measured. In addition,
these tri-atomic molecules participate
in the radiative heat transfer in
the atmosphere.
Unless another cooling medium can
dissipate this thermal emission by some
other mechanism, global warming will
persist. We can only minimize the rate of global warming and not eliminate
it as long as the energy conversion
from one form to another happens
with certain uncontrolled emission of
heat. This necessitates the reduction of
thermal emission and its constituents.
Minimizing the temperature of gaseous
emissions by simple methods will go a
long way toward minimizing the rate of
increase in atmospheric temperatures.
REDUCTION OF CO2
Extractive metallurgists can reduce
any oxide compound to its elemental
form. Examples of this are the making
of iron from iron oxides, aluminum
from aluminum oxides, and hydrogen
from hydrogen oxide (or water). Carbon
dioxide is just like any other oxide
and can be reduced to its respective elements
by applied energy, a process that
could minimize the amount of CO2 released
in the air and result in improved
fuel self sufficiency. During the CO2
Reduction Metallurgy Symposium at
the TMS 2008 Annual Meeting, metallurgists
will discuss how to accomplish
these goals in a cost-effective manner
using all available energy sources, including
solar and stored energy in the
form of presently land-filled municipal
wastes.
Reductions require some form of
energy to overcome the negative free
energy of formation of the compound
oxides. Such reductions into fuels are
better than just capturing CO2 and
pushing it back into the ground. The
second option of CO2 reduction metallurgy
is using energy from various
alternate sources in making materials.
In addition, the process development in
improving energy efficiency minimizes
CO2 emissions by reducing the use of
carbonaceous fuel. Energy efficiency
improvements also include recycling the oxidative abilities of contained oxygen
in CO2. All these approaches are
complimentary to each other.
SYMPOSIUM SUPPORTERS
Worldwide commitments to reduce
CO2 emissions to pre-1990 levels in the
next 12 to 13 years pose a formidable
challenge. To make this goal economically
feasible, new technologies such
as those that may come from this symposium
are essential.
Approaches to CO2 emission reductions
in metal production by improved
energy efficiency in life-cycle fuel use,
reduction in carbonate based flux/raw
material usage, and thermodynamically
feasible reactions leading to lower
emissions are all part of this program.
The symposium attracted the following
members of the scientific community
as co-sponsors: the National Materials
Advisory Board; the Metallurgical
Society of the Canadian Institute of
Mining Metallurgy and Petroleum;
the American Iron and Steel Institute
(AISI); the TMS Light Metals and Extraction
& Processing Divisions, Reactive
Metals Committee, and Recycling
and Environmental Technologies Committee.
This symposium, the first of its kind
in applying extractive metallurgy techniques,
is complimentary to several
international conferences on CO2 utilization
(there have been eight such
symposia so far, the last of which was
held in Oslo, Norway in 2005),2 and
minor symposia on the subject by the
American Chemical Society, American
Institute of Chemical Engineers, Electrochemical
Society, etc., in promoting
pertinent know-how in solving these
global concerns. Study of the conversion
of CO2 to other chemicals started
in the 19th century and was revived
several times. Considerable research
work has been reported related to space
travel and CO2 conversion.
The upcoming symposium is divided
into three major sessions: mechanisms,
ferrous metallurgy, and electrolytic approaches.
Typically, TMS symposia
are reviewed in JOM after the fact,
with summaries of the papers presented.
This article provides a view of the
planned presentations, compiled from
the abstracts and extracts from the
papers submitted by the authors. The symposium proceedings in CD and
book form will be available during the
conference.3
The organizing team started with
four and is down to the following three:
Neale Neelameggham, US Magnesium
LLC; Masao Suzuki, AI Tech Associates;
and Ramana Reddy, University
of Alabama. (The organizers are sorry
to note the unexpected and unfortunate
demise of our fourth organizer, Ralph
Harris, McGill University, during July
2007. Harris facilitated the co-sponsorship
of the Metallurgical Society of the
Canadian Institute of Mining Metallurgy
and Petroleum.) One of the three
sessions related to ferrous metallurgy
is geared toward addressing the CO2
reduction issues in the largest tonnage
metal—iron. The AISI co-sponsorship
was facilitated by Pinakin Chaubal,
Mittal Steel and Larry Kavanaugh,
AISI.
These CO2 reduction topics will alternate
between chemical reduction of
CO2 as an oxide as well as physical reduction
of the emission by using less
carbon—thus less CO2, and/or less energy
utilization.
TOPICS COVERED
Keynote Address
The symposium will begin with a
keynote address by Meyer Steinberg,
who is retired from Brookhaven National Laboratory. He is the co-author
of a detailed treatise Greenhouse Gas
Carbon Dioxide Mitigation: Science
and Technology,4 based on more than
30 years each of expertise in this field
by him and his co-author M. Halmann.
Steinberg, who started his career as a
Manhattan Project scientist, notes that
mitigating global greenhouse effect
while maintaining a fossil fuel economy
requires improving the efficiency
of conversion and utilization of fossil
fuels, use of high-hydrogen-content
fossil fuels, decarbonization of fossil
fuels, and sequestration of carbon and
CO2 applied to all energy-consuming
sectors of the economy including electric
power generation, industrial domestic
heating, materials production,
and transportation.
Steinberg’s review will cover the
principles of removal and recovery
from power plant stacks, the oceanographic
and geological disposal of CO2,
and the conversion of CO2 to gaseous
and liquid transportation fuels.
Mechanisms
The keynote speech will be followed
by five papers from the session on
mechanisms, co-chaired by Ray Peterson,
Aleris International, and Mahesh
Jha, Department of Energy.
University of Utah professors Michael
Moats, Jan Miller, and Wlodzimierz
Zmierczak will consider chemical utilization of sequestered CO2 as a
booster of the hydrogen economy. It is
noted that hydrogen in a pure hydrogen
economy would be like natural gas
in today’s energy economy. Unfortunately,
hydrogen’s physical properties
are unsuited to the energy market’s requirements
in terms of packaging, storage,
transfer, and delivery.
Site
|
Program
|
Savings
|
|
1
|
Technology only
|
-4%
|
|
2
|
Technology only
|
3%
|
|
3
|
People Only
|
16%
|
|
4
|
Comprehensive
|
23%
|
|
In this paper, a hybrid energy economy
that packages hydrogen chemically
on carbon atoms from various sources
including recycled CO2 is introduced
and discussed. For this new hybrid energy
economy to become a sustainable
reality, the ability to recycle CO2 and
attach hydrogen to create a usable energy
product, such as dimethyl ether, is
needed. Dimethyl ether is recognized
as a potential next-generation, environmentally
benign material for energy
storage and distribution. To maximize
the sustainability of the proposed hybrid
energy economy, green hydrogen
will be utilized, created by the electrolysis
of water powered by renewable
energy sources, such as solar, wind, or
geothermal heat.
Phillip A. Armstrong and Charles
A. Lewinsohn of Air Products (Allentown,
Pennsylvania) and John Gordon,
Ceramatec Inc. (Salt Lake City, Utah),
will discuss systems based on mixed
conductive ceramics. In particular, ceramics
conductive to oxygen ions and
electrons, for reducing CO2 emissions
from metallurgical production. Membranes
based on these mixed conducting
properties enable commercial viability
of several attractive metallurgical
processes. One of these processes
utilizes modular, ceramic-membrane
structures for the generation of pure
oxygen, in tonnage quantities. It is noted
that membrane-based systems offer
significant capital savings and operating
efficiencies in various metallurgical
processes.
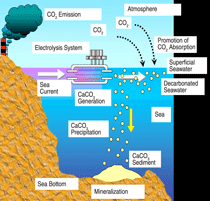
Katsuyoshi Tatenuma, Kaken Inc. (Ibaraki, Japan), proposes to reduce
global atmospheric CO2 by isolating
surplus CO2 from the biosphere, permanently
fixing it as an insoluble mineral
onto the sea bottom. This method
is based on the concept that an insoluble
carbonate mineral (CaCO3) can
be formed by direct electrolysis of the
seawater,
Ca2+ + 2HCO3- + OH-
= CaCO3(insoluble) + CO32-(soluble)
+ H+ + H2O
(this is not the coral reef reaction),
and is directly disposed by itself onto
the sea bottom. By this treatment, a
concentration of carbonate is reduced
in the seawater. The absorption capacity
of atmospheric CO2 is therefore
increased as a result of chemical equilibrium
between the ocean surface and
the atmosphere. The proposed method,
without any additives or generation of
secondary wastes or CO2 itself, and disturbance
of the environmental balance
has potential as a green-oriented method
with an ability to resolve fundamentally
the problem of global warming.
The schematic of the process is shown
in Figure 1.
Maria Salazar-Villalpando and Todd
Gardner, National Energy Technology
Laboratory (NETL), will talk on CO2 reduction by dry methane reforming
over hex aluminates. Methane reforming
using CO2 has been of interest for
many years because CO2 provides a
source of clean oxygen. Coal power or
metallurgical plants have wasted heat
streams that can be utilized in CO2 reduction.
The product of this reaction
is syngas, which could generate electrical
power in a single-oxide fuel cell
or used in the production of synthetic
fuels. Hexaluminate catalysts prepared
at NETL may represent a product that
can be utilized for the conversion of
CO2 to syngas. A series of BaNixAl12–y
O19–z catalysts were prepared by coprecipitation
followed by calcination
at 1,400°C. Reactions were carried out
to determine catalyst performance and
catalyst characterization was conducted
to determine surface area, pore size,
catalyst phases, and structures. Moreover,
catalyst characterization analysis
of three samples (y = 0.2, 0.6, and 1.0
in BaNixAl12–y O19–z) was performed by
extended x-ray absorption fine structure
and temperature programmed reduction.
Mark Berkley, Jim Sarvinis, Jason
Berzansky, and David Clarry, of Hatch
Associates (Brisbane, Australia), give
an in-depth overview of existing methods
in the CO2 capture and sequestration
implications for the metals industry.
Technologies developed to sequester
CO2 or use CO2 for enhanced fossil
fuel recovery are currently in operation.
Taxation regimes and CO2 credit trading
are becoming drivers for a number
of projects. Some mining companies
have also identified an opportunity to
sequester CO2 in their by-products; recent
work by Alcoa in using by-product
alkalinity is a prime example. In addition
to process plant emissions, mining
companies are becoming increasingly
aware of their overall carbon footprint,
including CO2 generated during the
production of power they use. New
power plant designs in various stages
of development include CO2 separation
and sequestration techniques. This paper
summarizes the state-of-the-art for
these technologies, including integrated
gasification combined cycle power
plants. A concept to use the gasification
products as reductants in metallurgical
processes is also described. The
relevance of technology from power
plant designs with post-combustion
capture to the processing of off-gas generated in metallurgical facilities is
also reviewed.
The authors touch upon several
physical and chemical methods of concentrating
and separating CO2 from
the flue gas, assuming state-of-the-art
methods. This is still a fertile field for
metallurgists.
Ferrous Metallurgy
The ferrous metallurgy session on
CO2 reduction metallurgy, co-chaired
by Larry Kavanaugh (AISI) and K.
Mondal (Southern Illinois University),
will feature the following topics.
Jean-Pierre Birat, Arcelor-Mittal Research
(Maizicres-les-Metz, France) will present
the EU work on CO2 in the steel industry
and the ultra-low CO2 steelmaking
program. The European steel industry
has been engaged since 2004 in an extensive,
five-year, 50 million euro program
to develop breakthrough process
technologies to produce steel with a reduction
of specific CO2 emissions by at
least a factor of 2. This program, called
ultra-low CO2 steelmaking, will deliver
several process route concepts that meet
this target and are ready for scaling up
to a commercial size pilot by 2009. After
screening a large number of potential
routes in extensive future studies,
the program now focuses on five concepts.
The oxygen blast furnace with
top gas recycling and carbon capture
and sequestration (CCS); a bath smelting
reduction process, also using pure
oxygen and CCS; a new direct reduction,
also oxygen and CCS-based, and
two processes to carry out the electrolysis
of iron ore; they will now be tested
at fairly large scales. The approach may
be of interest to other metallurgies than
steelmaking.
| Reaction
|
ΔG° (kJ/mol)
|
E°V
|
| CO2 + 2 H+ + 2 e– → HCOOH |
22.06
|
-0.114
|
| CO2 + 2 H+ + 2 e– → CO + H2O
|
20.06
|
-0.104
|
| CO2 + 4 H+ + 4 e– → HCHO + H2O
|
40.91
|
-0.106
|
| CO2 + 6 H+ + 6 e– → CH3OH + H2O
|
-18.08
|
-0.031
|
*CO2 and CO are gases. All other substances are aqueous solutions. |
R. Malti Goel, special advisor, Department
of Science and Technology,
Government of India, will talk about
the recent developments in technology
management for reducing of CO2 emissions
in the metal industry. The ferrous
and non-ferrous industries in India are
undergoing a transition to meet growing
demand and a new thrust toward
R&D is mounting. This paper describes
the recent developments in technology
management, the importance of R&D,
and case studies on adoption of energy efficient
and CO2 reduction technologies
in the metal industry in India.
James Evans, University of California,
Berkeley, and Brian Wildey, Pacific
Consolidated Industries (Riverside,
California) will present the impact on
greenhouse gas emissions of a switch
from carbon to hydrogen as the principal
reducing agent in producing metals.
The authors point out that carbon
has been the principal reducing agent
in producing metals for centuries. Carbon
has been an inexpensive reducing
agent, but also an effective one, as any
undergraduate who knows his or her
Ellingham diagrams is aware. Even in
the production of aluminum, an electrolytic
process, carbon consumed at
the anodes serves to reduce the voltage
of the Hall–Héroult cell. The interest
in reducing the amount of anthropogenic
CO2 has led to this examination
of whether a switch to hydrogen as a
major reductant is feasible and economical.
The main source of hydrogen
is natural gas and its production entails
the generation of CO2; however, that
CO2 is more easily captured than, say,
the CO2 leaving an iron blast furnace.
Calculations and speculations lead
to conclusions about whether there is
significant benefit to be obtained from
such a radical change in our way of
producing metals and the approximate
cost of such change.
Von Richards, Simon Lekakh,
Charles Rawlins, and Kent Peaslee,
of the University of Missouri at Rolla,
will discuss the subject of sequestration
of CO2 by steelmaking slag—inclusive
of the process phenomena and
reactor study. Steelmaking processes
generate CO2 air emissions and a slag
co-product. This project developed a
functional sequestration system using
steelmaking slag to permanently capture
CO2 emitted in steelmaking offgas.
A possible parallel benefit of this process
would be rapid chemical stabilization
of the slag minerals with reducing
swelling or leaching. The authors will
be presenting the results of the project,
including mineralogical and structural
features of carbon sequestration with
steelmaking slag, mathematical modeling
of reaction phenomena using a
modified shrinking core model, Metsim
modeling of several possible industrial
applications, a thermo-gravimetrical
study of the reaction between slags and
different gases, and design and testing
for a lab-scale apparatus consisting of
two reactors.
Jon Feldman, Edmund Smith, Stephen
Gale, and David Clarry of Hatch
present a management technique used
at a Canadian steel plant leading to impressive
results. In 2006, independent
steelmaker Algoma Steel Inc. (Ontario,
Canada) embarked on an ambitious
initiative to become “Best in Class in
Energy Management.” The initiative
focused on identifying energy/greenhouse
gas management improvements
leading to low-implementation cost approaches
to energy savings.
The authors note that the key components
of the approach were: review
site energy management practices and
benchmark internationally; review best
technical practices and benchmark
against public energy intensity data;
conduct a site-wide energy assessment
and physical review of operating areas;
facilitate on-site workshops with staff
to prioritize savings opportunities and
identify and remove implementation
barriers; and develop an energy management
action plan for implementation
of improvements.
Most organizations can produce,
with relative ease, a list of ideas for
saving energy, but implementing them
is the challenge. This paper addresses
these issues as well as the profitability
of the energy efficiency improvements
and the consequences for greenhouse
gas emissions. Table I shows the range
of benefits accrued from differing levels
of project approaches.
Chenguang Bai, Liangying Wen,
and Feng Xia from Chongqing University,
China, present a technique for
the reduction of Ti-V-magnetite with
microwave energy, which effectively
minimizes CO2 emissions in the process.
The relationship between carbon
consumption and microwave energy
consumption for the magnetite reduction
process was studied. At different
microwave power levels, the carbon usage
per unit of ore is different. While
the output of power and irradiation
time increase, carbon consumption
decreases. The results prove that microwave
energy is a good resource that
can be used in magnetite extraction to
reduce CO2 emission.
Electrolytic Methods
The use of electrolytic methods is the
logical and thermodynamically analyzed
choice for CO2 reduction metallurgy.
This session is co-chaired by D.
Sadoway of the Massachusetts Institute
of Technology (MIT) and J. Hryn
(Praxair; Danbury, Connecticut).
This session will start with a discussion
by Sadoway of carbon-free metals
extraction by molten oxide electrolysis.
He notes that molten oxide electrolysis
(MOE), which is the electrolytic
decomposition of molten metal-oxide
into liquid metal and oxygen gas, represents
an improvement over today’s
carbon-intensive thermochemical reduction
processes for metal production.
For MOE, the feedstock can be
concentrate derived from ore or from
hazardous waste (such as chromate
sludge). The process avoids the use of
consumable carbon anodes and thus
eliminates greenhouse-gas emissions
as the by-product of the metal-recovery
step. The concept applies to a variety
of chemistries including titanium, iron,
and ferroalloys. The electrochemistry of
multi-component oxide melts from the
family FeO–SiO2–TiO2–Al2O3–MgO–
CaO has been studied by voltammetry
at temperatures up to 1,750°C. Galvanostatic
electrolysis in laboratory-scale
cells operating with a variety of feedstocks
has demonstrated the extraction
of liquid iron, ferrosilicon, and titanium
with the simultaneous co-generation of
oxygen.
The rest of this session will cover effects
of electrolytic reduction of CO2
using electron transfers of two to eight
electrons making different combinations
of (alloys of) carbon, hydrogen,
and oxygen from CO2 and H2O.
Melvin Miles of the University of La
Verne (La Verne, California) will cover the
subject of an electrochemical reduction
of CO2 with a six electron transfer. He
notes that considerable research has
been conducted on the anodic oxidation
of methanol in fuel cells. The electrochemical
reduction of CO2 in aqueous
solutions to form methanol is the
same reaction in reverse (i.e., CO2 + 6
H+ + 6 e- = CH3OH + H2O). The same
catalysts and conditions may operate
for both reaction directions. This CO2
reduction reaction, however, must be
able to compete with the electrochemical
reduction of water to form hydrogen
gas. Thermodynamically, the reduction
of CO2 to form CH3OH is slightly more
favorable than the reduction of water.
Kinetically, CO2 reduction can be favored
by electrodes that are poor catalysts
for water reduction such as Mo,
Cu, In, Sn, and Sb. The use of nearly
neutral electrolytes rather than acidic
electrolytes makes the reduction of
CO2 kinetically more favorable. The
thermodynamics of various reaction
steps will be presented.
The author provides the electromagnetic
fields of varying CO2–H2O electrolytic
reactions, as shown in Table II.
Neale Neelameggham of US Magnesium
LLC (Salt Lake City, Utah) will
discuss the fundamentals of soda fuel
cycle metallurgy. Processes for generating
alloys of carbon and hydrogen
by reducing the CO2 and water mixture
(carbonated water or water vapor)
called soda, to easily stored fuels are
normally endothermic. The processes
are similar to the generation of hydrogen
alone from water, which is also
endothermic. But hydrogen is more
difficult to store than compounds (or
alloys) of carbon and hydrogen. This
recycling of CO2 and water is possible
by using other forms of energy by innovative
carbon capture methods.
Thermodynamic diagrams developed
for soda (CO2 and H2O)–metal reactions
provide hints for using low-cost
stored energy in un-recycled metallic
waste for thermal conversion. The
soda-to-fuel using metals reaction is
written as
m CO2 + n H2O + p M
= CmH2nOy + MpO(2m + n – y)
Choices for conditions for the electrochemical
approaches (using solar,
wind, and hydropower) are also discussed.
The product mix will be a
function of the reactant stoichiometry
between CO2 and H2O. It is pointed out
that this stoichiometric control may be
better in non-aqueous electrolytes.
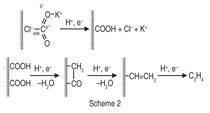
Kotaro Ogura of Yamaguchi University
discusses his solution of how to
carry out the electrocatalytic reduction
of CO2 to C2H4 at a gas/solution/metal
interface. He proposes that the problem
of greenhouse effect can be largely mitigated
if CO2 can be recycled for use
as fuels or chemicals. Although various
processes for converting CO2 to valuable
compounds have been proposed, it
is important that the conversion process
be feasible under mild conditions. This
is because the secondary generation of
CO2 is feared if high energy is required
and supplied with a fossil fuel. Ogura’s
research developed the electrochemical
reduction process of CO2 to C2H4
which occurs at a three-phase (gas/solution/
metal) interface in concentrated
potassium halide solutions. The input
energy required for this process was
supplied by a solar cell or a commercial
power source. In the closed circulation
system, the conversion efficiency of
CO2 increased with an increase of input
electric charge to reach 100%, and the
maximum selectivity for the formation
of C2H4 was more than 80%. Ogura’s
mechanism of CO2–H2O electrolysis
making ethylene is shown in Figure 2.
Mingming Zhang and Ramana Reddy,
from the University of Alabama,
have been pioneering the advantages
of green production of light metals in
ionic liquid electrolytes. Carbon dioxide
is one of the most common by-products
of present-day light metals production.
The authors propose a novel light
metals production process using ionic
liquid electrolytes. This process has
significant advantages compared with
traditional high-temperature processes,
such as zero CO2 and CF4 emission;
low energy consumption due to the
low temperature operation; and nonconsumable
electrodes. Two types of
anode materials (graphite and extruded
carbon) were assessed in an electrolysis
experiment. The results showed
that both anode materials were stable
(negligible weight loss). However, the
extruded carbon was found to be superior
to graphite in terms of facilitating
higher current density and current
efficiency. The calculated energy consumption
for aluminum production was
less than 5 kWh/kg at up to 350 A/m2.
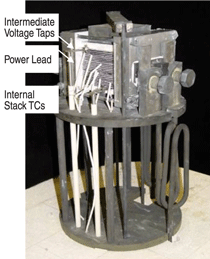
Joseph Hartvigsen, S. Elangovan,
Lyman Frost, and Anthony Nickens
of Ceramatec (Salt Lake City, Utah),
and Carl Stoots, James O’Brien, and
J. Herring of Idaho National Laboratory
(INL) will present their work on
CO2 recycling by high-temperature coelectrolysis
and hydrocarbon synthesis.
The raw material for synfuel production
is syngas, a mixture of H2 and CO.
Ceramatec and INL have demonstrated
conversion of CO2 and steam to syngas
by high-temperature solid oxide
co-electrolysis, followed by catalytic
conversion of the syngas to a hydrocarbon
fuel. This is a controlled electrolysis
without using much excess CO2 or
excess H2O in making synthesis gas
followed by demonstrated conversion
into hydrocarbon fuel. Carbon dioxide
recovered from concentrated sources,
such as metallurgical reduction furnaces,
cement kilns, and fossil fuel power
plants can provide a valuable feedstock
for a synthetic fuels industry based on
energy from nuclear, solar, wind, and
hydropower sources. This electrolysis
technology is the reverse mechanism
of conventional solid oxide fuel cell
technology.
Wherever a source of high-temperature
process heat is available, such as
from an advanced high-temperature nuclear
reactor or solar dish concentrator,
the endothermic electrolysis reactions
can utilize both thermal and electrical
inputs in such a way that the conversion
efficiency within the cell is 100%
followed by conversion to synfuel.
Widespread implementation of such
synfuel production from CO2 will enable
a much larger reliance on intermittent
renewable energy than can be accommodated
by conventional electric
demand profiles. Their ten cell stack of
a solid oxide CO2-steam electrolyzer
making syngas is shown in Figure 3.
Kanchan Mondal of Southern Illinois
University will present his mechanistic
concepts on CO2 reduction by
nanoscale galvanic couples. He notes
that methanol, lower hydrocarbons,
CO, and HCOOH have been formed
by the reduction of CO2 with H2. Uncatalyzed
electro-reduction requires a
significant overvoltage. An alternate
route has been conceptualized. Production
of hydrogen from water can
be achieved by chemical oxidation of
a metal in water followed by oxidation
of the hydronium ion by the released
electrons to form hydrogen radicals. It
is hypothesized that circumventing the
problem of the formation of hydrogen
molecules is essential while having the
hydrogen radical react with CO2 forming
methanol, etc. This may be possible
by a bimetallic catalyst acting as a galvanic
couple, wherein one metal acts as
the electron donor for the production of
hydrogen radicals while the other acts
as a catalyst for the reduction of CO2.
This may enhance the reaction rates.
The concept circumvents the need for
an external electric field unlike the
electrochemical process.
Kandasamy Subramanian, Krishnasamy
Asokan, and Govindarajan Gomathi
of Central Electrochemical Research
Institute (Karaikudi, India), will
discuss their experience in carrying out
the two-electron transfer reduction of
CO2 to formate ion in an electrochemical
membrane reactor in KHCO3 buffer
solutions.
An electrochemical reactor with anode
and cathode chambers separated
by a composite perfluoro polymer cation
exchange membrane was designed,
fabricated, and used for the reduction
of dissolved CO2 to formate under
ambient conditions. The flow reactor
enhanced the mass transfer of carbon
dioxide compared to the batch reactor.
Experiments were conducted using two
different membranes—Nafion 961 and
Nafion 430. Optimum values of flow
rate and current density were evaluated
for the formation of formate in aqueous
KHCO3 buffer solutions. The efficacy
of various cathode materials (Cd, Pb,
Sn, and Zn plated stainless steel woven
mesh) for the electro-reduction of CO2 is also reported.
Each session will end with a panel
discussion to get input from other
members, whose comments and contributions
to this field will be worthwhile.
I would like to end this synopsis with
a quote (author unknown) “Along the
way there were those who knew all the
reasons these things couldn’t be done.
Fortunately there were those who knew
enough not to listen.”
REFERENCES
1. R. Neelameggham, “Fossilize CO2–Make Fuels, A
Green and Black Paper on Soda-Fuel Cycle” (private
communications, August 2006).
2. Eighth International Conference on Carbon Dioxide
Utilization (20–23 June 2005, Oslo, Norway), www.kjemi.uio.no/iccduviii/Final_Schedule.pdf.
3. Neale R. Neelameggham and Ramana G Reddy,
editors, Proceedings of Carbon Dioxide Reduction
Metallurgy Symposium (Warrendale, PA: TMS, 2008).
4. Martin M. Halmann and M. Steinberg, Greenhouse
Gas Carbon Dioxide Mitigation: Science and Technology (Boca Raton, FL: CRC Press LLC, 1999).
Neale R. Neelameggham is the technical development
scientist, US Magnesium LLC, 238 N 2200
W, Salt Lake City, UT 84116; rneelameggham@usmagnesium.com. Dr. Neelameggham is also the
JOM advisor from the Reactive Metals Committee
of the TMS Light Metals Division.
|