LATEST ISSUE |
||||
TMS QUICK LINKS: |
• TECHNICAL QUESTIONS • NEWS ROOM • ABOUT TMS • SITE MAP • CONTACT US |
JOM QUICK LINKS: |
• COVER GALLERY • CLASSIFIED ADS • SUBJECT INDEXES • AUTHORS KIT • ADVERTISE |
|
| Overview: Cobalt: Winning, Recycling, and Applications | Vol. 58, No.10, pp. 47-50 |
Cobalt—Its Recovery,
Recycling, and Application
Shijie Wang
| ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
Questions? Contact jom@tms.org. © 2006 The Minerals, Metals & Materials Society |
|
INTRODUCTION For years, cobalt was thought by some to be radioactive. This misconception may be attributable to the Planet of the Apes movies, which centered on a “divine bomb”—a nuclear missile with a cobalt casing. Another possible source of the cobalt misconception is that the radioactive isotope cobalt-60 is widely used in industrial applications and in hospital radiotherapy. Cobalt is, in fact, a metal that is stable, nonradioactive, and found in nature, while cobalt-60 (Co-60) is unstable, radioactive, and manmade.
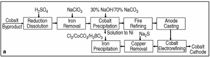
The name “cobalt” was derived from the German “Kobold,” meaning evil sprite. The interest in cobalt’s uses goes back to 2000 B.C. when cobalt was used by Egyptian artisans as a coloring agent. Cobalt salts have been used for centuries to produce brilliant and permanent blue colors in porcelain, glass, pottery, tiles, and enamels. After mining techniques became widely known in the 16th century through the works of Georgius Agricola, a German mineralogist, cobalt was supplied as raffre, a cobalt arsenide of sulfide ore that was roasted to yield a cobalt oxide. In 1735, a Swedish scientist, Georg Brandt, demonstrated that a blue color common in colored glass was caused by a new element, cobalt. Metallic cobalt was later established as an element by Torbern Bergman in 1780. In 1926, and again in 1929, a process based on the use of nickel peroxide for selective precipitation of cobalt from nickel electrolyte/sulfate-boric acid was studied, but was found to be unattractive. In 1942, the use of a sulfate-chloride electrolyte for nickel refining was introduced, thus paving the way for the successful development of a cobalt recovery process.1–3 Although the electro-cobalt recovered from the process described in the sidebar and shown in Figure Aa shows a very high purity of cobalt cathode (99.98% cobalt) commercially produced, processes for cobalt recovery are significantly changed by following hydrometallurgical lines. Solvent extraction-electrowinning (SXEW) technology is widely embraced in these changes (Figure Aa, b, c, d, and e) because of commercial needs and environmental reasons (i.e., eliminating fire refining). Since cobalt electrolyte is very sensitive to metallic impurities, in particular, iron, nickel, copper, magnesium, and manganese, solution purification must be effectively conducted prior to cobalt winning to meet an increasing demand for high-purity cobalt. Solution Purification Solvent extraction using selective P507 (similar to PC-88 and SME-418) and Cyanex 272 separates cobalt and nickel from both sulfate and chloride media, as shown in Reactions 11 and 12. Using selective D2EHPA, P204 (HR2PO4), Cyanex 302/301, Chelex 20, and Purolite S-930 at pH 2–5, SX strips zinc, iron, copper, and aluminum with sulfuric acid. LIX 841 (ketoxime reagent) can be used to selectively recover the nickel to a purified nickel electrolyte. With the ion exchange method, Lewatit TP 207 can effectively remove copper and zinc from nickel and cobalt solution. This type of cation exchange resin can also separate nickel from cobalt solution or vice versa. The newest Lewatit MonoPlus TP 207 is used in separating magnesium and calcium from nickel and cobalt solution as well as in nickel recovery.9 MRT10,11 SuperLig® 241 can be used to extract and polish nickel from a cobalt feedstock solution, which is very effective in separating nickel from cobalt when the Co/Ni ratio ranges up to 500–1,000:1. Also, SuperLig® 176 can be used for the combined removal of copper and Fe(III) together with nickel from the cobalt electrowinning electrolyte. For cobalt recovery in a copper sulfide ore process, copper is removed via electrowinning and iron, zinc, and more copper are precipitated by increasing the pH of the solution. Cobalt Winning Operation Due to the stresses inherent in cobalt metal, electrodeposited cobalt tends to peel from the cathode blank in electrowinning. To avoid this and maintain an adherent deposit in practice, cobalt is electrowon onto 6 mm thick stainless-steel blanks and allowed to grow around the edges of the blank.12 The cobalt metal produced can either be broken cathode or it can be cut in small pieces. Cobalt is also electrowon as “round” discs 2.53 cm in diameter, deposited onto stainless-steel mandrel sheets, or electrown directly as metallic powders. Dimensionally stable anodes (DSA) are used exclusively in cobalt chloride electrolytes. The anodes are bagged to collect the chlorine gas generated. Alloyed lead anodes are most widely used in sulfate medium. Electrowinning cobalt metal from sulfate electrolyte makes the winning operation possible in undivided cells and enables significant process changes to adapt the SX-EW technology from sulfides to laterites. During electrowinning, hydrogen evolution is a possible competitive side reaction that reduces metal deposition current efficiency. However, some laboratory findings13 indicated that “plating and dissolving simultaneously” within an oxidation system (i.e., presenting of residual chlorine or hydrogen peroxide in electrolyte) may be a root cause of the low current efficiency. In 1997, the U.S. annual consumption of cobalt was 9,200 tonnes and 22% of the cobalt consumed was recycled and recovered from alloy scrap, cemented carbide scrap, and spent catalysts. Cobalt resources can be maximized with its recovery from leaching solution, electrowinning bleed, smelting slag, sludge, and residues. Recycling of Spent Catalysts Gulf Chemical and Metallurgical Corporation processed Ni/Mo, Co/Mo, and Ni/Co/Mo/V catalysts in a 14 MW electric arc furnace (EAF). Alumina concentrate, produced after roasting and leaching the spent catalysts, is mixed with a reductant and melted in the EAF to produce a mixed metallic alloy containing 37–43% nickel and 12–17% cobalt, which is finally sold to nickel and cobalt refineries. Recycling of Alloy Scrap To recover strategic and critical metals from superalloy scrap (SAS), the former U.S. Bureau of Mines developed a process that utilized novel double membrane electrolytic cells (DMEC) to electrorefine SAS into high-purity cobalt and nickel cathodes.17 Integrated with copper cementation, iron SX, carbon adsorption, and cobalt SX operations, the DMEC cobalt cathode produced had a purity of >99.95% cobalt, above the purchase specification. Recovering from Cobalt-Bearing
Solutions Recovering from Sludge/
Residue Fluoborate technology,22 whose leaching process is expressed in Equation 16, was also identified as an effective lixiviate in treating the sludge at both room and elevated temperatures, where the oxidation reaction happened to solubilize the base metals in the fluoborate system. At temperatures below 417°C, cobalt exhibits a hexagonal-close-packed structure. Between 417°C and its melting point of 1,493°C, cobalt has an fcc structure. Cobalt is ferromagnetic and retains this property to 1,100°C. Cobalt has played an important part in the composition of nearly all the new alloys developed since the 19th century for cutting tools and wear resistance. The special properties of cobalt have been utilized in such applications as catalysts, paint dryers, polymerization promoters, and rechargeable battery chemicals. Some of them are described as follows.23–26 Superalloys are major applications where cobalt improves the strength, wear, and corrosion-resistant characteristics of alloys at elevated temperatures. The major uses of cobalt-based superalloys (45% cobalt) are in turbine blades for aircraft jet engines and in gas turbines for pipeline compressors. Hardfacing alloys are specialized applications where the cobalt-based alloy is used for machining very hard materials. In such applications the most important group of cobalt-based alloys is the stellite group, which contains cobalt, tungsten, chromium, and molybdenum as principal constituents. The hardfacing tools with cobalt alloy, known as stellite alloys (Co-Cr), invented by Haynes in 1907 and applied first in knives, provides greater resistance to wear, heat, impact, and corrosion. Magnets are another important application for metallic cobalt where it is used in the manufacture of various magnetic materials. The remarkable and unexpected magnetic properties of the alloy Fe2Co were announced by Weiss in 1912. Perhaps the most important of these properties is the permeability in medium fields; that is, for a magnetizing force of 50–600 gilberts per centimeter. Hard materials-carbides are an important application for cobalt metal powder which is used as a binder in the production of cemented tungsten carbides for heavy-duty and high-speed cutting tools. In cemented carbides (3–25% cobalt), cobalt provides a ductile bonding matrix for tungsten-carbide particles. The application of cobalt in catalysts is based on its property of multivalence (Co2+ and Co3+). A cobalt-molybdenumalumina compound is used as a catalyst in hydrogenation and for petroleum desulfurization. Cobalt metal is used in electroplating because of its attractive appearance, hardness, and resistance to oxidation. Radioactive Cobalt-60 was discovered by Glenn T. Seaborg and John Livingood at the University of California–Berkeley in the late 1930s. Cobalt-60, a radioactive isotope of cobalt, is an important source of gamma rays and is used to treat some forms of cancer and as medical tracer. Cobalt-based batteries are another extraordinary application where the use of cobalt in rechargeable batteries grew enormously between 1995 and 2000. Batteries are electrochemical devices that convert chemical energy into electrical energy with an anode and a cathode. The addition of cobalt to the electrodes substantially enhanced the cell’s life, increased hydride thermodynamic stability, and inhibited corrosion. The increasing use of cobalt in rechargeable batteries for electric vehicle applications is expected to increase the use of cobalt even further. A major shift to hybrid-electric vehicles in automotive technology will dramatically increase the demand for cobalt-based batteries. Newly emerging demand combined with increases in cobalt use for other types of turbine engines and gas-to-liquid catalysts is underpinning the recent growth in cobalt demand and is expected to drive future cobalt consumption to unprecedented levels. Based on its unique properties, cobalt is useful in applications that utilize its magnetic properties, corrosion resistance, wear resistance, and its strength at elevated temperatures. The blossoming of a wide variety of new applications, in addition to the old ones, will assure cobalt’s place in metallurgy for many years to come. 1. L.S. Renzoni, U.S. patent 2,367,239 (1945). Shijie Wang is senior engineer at Kennecott Utah Copper Corp. in Magna, Utah. For more information, contact Shijie Wang, Kennecott Utah Copper Corp., 11600 W 2100 South, P.O. Box 6001, Magna, UT 84044; (801) 569-6747; fax (801) 569-6753; e-mail wangs@kennecott.com. |