|
It is well known that titanium has
properties attractive to the aerospace
and other industries, and that its applications
are limited due to its high cost.
This overview will provide those not directly
involved with titanium an explanation
of why titanium is an attractive
material, with aerospace being a primary
focus. A brief summary of titanium
applications and some of its unique
properties will also be discussed.
INTRODUCTION
The primary attributes that make titanium
an attractive material include an
excellent strength-to-weight ratio, providing
weight savings attractive to the
aerospace and petrochemical industries;
corrosion resistance, particularly appealing
to the aerospace, chemical, petrochemical
and architectural industries;
and biological compatibility, of interest
to the medical industry. The chemical
industry is the largest user of titanium
due to its excellent corrosion resistance,
particularly in the presence of oxidizing
acids. The aerospace industry is the
next largest user, primarily due to its elevated
(and cryogenic) temperature capabilities
and weight savings due to its
high strength and low density; with increased
use of polymeric graphite fiber
reinforced composites on aircraft, the
low coefficient of thermal expansion is
also an important factor. The ballistic
properties of titanium are also excellent
on a density-normalized basis. Highlights
of titanium applications in other
areas will be briefly discussed.
| HOW WOULD YOU... |
…describe the overall significance
of this paper?
The intent is to provide those who
are not familiar with titanium and
those with just a little knowledge a
perspective on some of the unique
aspects and advantages of titanium.
…describe this work to a
materials science and engineering
professional with no experience in
your technical specialty?
The intent would be to explain what
is unique about titanium and the
types of applications where titanium
would offer advantages over other
materials.
…describe this work to a
layperson?
It provides a basic understanding of
the unique aspects of titanium and
describes the types of applications
where titanium should be used. |
RATIONALE FOR TITANIUM USAGE
Weight Savings
The high strength and low density of
titanium (~40% lower than that of steel)
provide many opportunities for weight
savings. The best example of this is its
use on the landing gear of the Boeing
777 and 787 aircraft and the Airbus
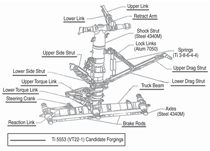
A380. Figure 1 shows the landing gear
on the 777 aircraft.1 All of the labeled
parts are fabricated from Ti-10V-2Fe-
3Al. This alloy is used at a minimum
tensile strength of 1,193 MPa; it is used
in replacement of a high-strength lowalloy
steel, 4340M, which is used at
1,930 MPa. This substitution resulted
in a weight savings of over 580 kg.1 The
Boeing 787 used the next-generation
high-strength titanium alloy, Ti-5Al-
5V-5Mo-3Cr, which has slightly higher
strength and some processing advantagesThe use of titanium in landing
gear structure should also significantly
reduce the landing gear maintenance
costs due to its corrosion resistance.
The low density and high strength
make it very attractive for reciprocating
parts, such as connecting rods for automotive
applications. Again, the price
is too high for family vehicles but the
U.S. Department of Energy is investing
in a substantial effort to make titanium
components for automobiles and trucks
affordable. (Titanium is successfully
utilized for high-end racing cars, where
cost is not that much of an issue.)
Space Limitations
This application does not come up
often, but it is an important one. The
best example for this is the landing gear
beam used on the 737, 747, and 757.
This component, running between the
wing and fuselage, supports the landing
gear. Other Boeing aircraft utilize
an aluminum alloy for this application,
but for the above aircraft the loading is
higher and the aluminum structure will
not fit within the envelope of the wing.
An aluminum alloy would be the preferred
option as it is much lower in cost.
Steel would be another option, but that
would be higher weight.
Operating Temperature
The structure in the engine and exhaust
areas operates at elevated temperature,
so the primary options are
titanium- or nickel-base alloys; again,
the nickel alloys would add significant
weight. Titanium engine alloys are used
up to about 600°C. There are applications,
such as the plug and nozzle (Figure
2), which experience temperatures
higher than this for short times during
certain operating conditions. The temperature
limitation for titanium alloys, other than specialized engine alloys, is
about 540°C. Above this temperature
oxygen contamination becomes an issue,
embrittling the surface. Titanium
is also used at cryogenic temperatures
for structures such as impellors for
rocket engines.
Corrosion Resistance
Titanium has a very tenacious nascent
oxide which forms instantly
upon exposure to air. This oxide is
the reason for the excellent corrosion
resistance. Corrosion is not a factor
for titanium in an aerospace environment.
Titanium does not pit, which in
the author’s opinion is the rationale
for the excellent service experience.
In service, aluminum and steel alloys
will eventually form corrosion pits,
which serve as stress risers which will
then initiate stress corrosion or fatigue
cracks. This does not happen with titanium.
This corrosion resistance carries
through to the chemical, petrochemical,
pulp, paper, and architectural industries.
Titanium and its alloys have
excellent resistance under most oxidizing,
neutral, and inhibited reducing
conditions. It is also corrosion resistant
within the human body. Biocompatibility
is also excellent; it is used for
prosthetic devices and bone will grow
into properly designed titanium structures.
Commercially pure titanium is
also being used for exterior architectural
applications, a practice started in
Japan. It is used for exterior surfaces as
it will never require any maintenance.
The most famous of these is its use on
the exterior of the Guggenheim Art
Museum in Bilbao, Spain.
Composites Compatibility
Titanium is compatible with the
graphite fibers in the polymeric composites.
There is high galvanic potential
between aluminum and graphite,
and if the aluminum comes into contact
with the graphite in the presence of
moisture the aluminum would be corroded
away. It can be isolated from the
composite by methods such as a layer
of fi berglass, but in areas that are difficult to inspect and diffi cult to replace,
titanium is used as a conservative approach.
In addition, the coeffi cient of
thermal expansion (CTE) of titanium,
while higher than that of graphite, is
much lower than that of aluminum.
Even in the operating temperature
range of fuselage structure, about
–60°C at cruise to +55°C on a hot day,
the difference in CTE using aluminum
structure attached to the composite
would result in very high loading. This
is not an issue with titanium structure.
Obviously, the longer the component,
the bigger the issue would be for utilizing
aluminum.
Low Modulus
The primary area where this is important
is in the replacement of steel
springs. With the modulus being about
half that of steel, only half the number
of coils are required. That in conjunction
with the high strength and density
being about 60% of that of steel could
ideally result in a weight savings of
about 70% of that of a steel spring.
In addition, the titanium offers much
superior corrosion resistance, reducing
maintenance costs.
Armor
Titanium has excellent ballistic
resistance and provides a 15–35%
weight savings when compared to steel
or aluminum armor for the same ballistic
protection at areal densities of
interest, which has resulted in substantial
weight savings on military ground
combat vehicles. Lighter vehicles have
better transportability and maneuverability.
The excellent corrosion resistance,
low ferromagnetism, and compatibility
with composites also provide
significant benefi ts. Two programs that
use titanium in upgraded vehicles are
the Bradley Infantry Fighting Vehicle
(Figure 3) and Abrams Main Battle
Tank.2 The relatively high cost of titanium
has been successfully mitigated
by using plate produced from electronbeam,
cold hearth, single melt ingot.3
UNIQUE ATTRIBUTES OF TITANIUM
General corrosion resistance has already
been discussed. With regard to
stress-corrosion cracking (SCC), commercially
pure and most titanium alloys
are virtually immune unless there
is a fresh, sharp crack in the presence
of stress. If the titanium is cracked in
air, the protective oxide will immediately
re-form, and SCC may not occur.
If the crack is initiated in sea water,
for instance, then SCC could occur on
certain high-strength alloys or high
oxygen grades of commercially pure
titanium. Even here, the SCC may
be mitigated if the part is not loaded
immediately. Dawson and Pelloux4
showed that fatigue crack growth of
Ti-6Al-6V-2Sn can be reduced when
tested at a low frequency as long as
the stress intensity is below that of
the stress corrosion threshold. This is
attributed to re-passivation (re-formation
of the oxide) in the sea water at
the lower frequency whereas there is
insufficient time for this to occur at
higher frequencies.
The modulus of ß-alloys can be altered
significantly. Ti-15V-3Cr-3Al-3Sn
with 60% cold work had a tensile
strength of ~1,070 MPa with a modulus
of ~76–83 GPa. When aged at 480°C
the strength and modulus were ~1,515
MPa and 103 GPa, respectively. Titanium
alloys containing Nb, Zr, and Ta,
referred to as gum metal, developed
for the medical industry, have elastic
moduli as low as 40–50 GPa depending
on orientation and processing.
These moduli are close to that of bone,
making it ideal for prosthetic applications.
Cold work decreases the modulus
while increasing the strength.5
The crystallographic texture of
the hexagonal close-packed (HCP)
a-phase can have a very significant
effect on properties in different directions.
Larson6 modeled the modulus of
a single crystal of commercially pure
titanium and determined that when
stressed along the basal pole the modulus
is ~144 GPa, but when stressed
orthogonal to the basal pole it is ~ 96
GPa. Differences in ultimate tensile
strength, which are also an indicator
of crystallographic texture, between
the longitudinal and transverse direction
of about 205 MPa have recently
been observed for rolled strip, with
continuous rolling in one direction
which can result in a strong texture.
The Bauschinger effect, while not
necessarily unique, seems to have a
stronger effect in titanium alloys than
other alloy systems. It is attributed to
the limited number of slip systems in
hexagonal close-packed (HCP) low
temperature α-phase. If a tensile specimen
is pulled in tension and the test
is stopped prior to failure, and a compression
specimen is taken from the
gage length of the tensile specimen, a
significant drop in the yield strength
is observed. A tensile strain of 0.5%
at room temperature can reduce the
compression yield by 30%. This is
attributed to the dislocations in the
material going in the reverse direction
following the same slip path, meaning
dislocation barriers do not have
to be overcome in the early stages of
deformation. The same phenomenon
is observed if one strains a compression
specimen and then pulls a tensile
from its gage length. This effect can
be eliminated or mitigated by forming
at elevated temperature, or subsequent
annealing. Consequently, at least in the
aerospace industry, when a titanium
part is formed, it is subsequently annealed
to avoid this large yield reduction.
It does not affect ultimate tensile
strength.
Solid metal embrittlement has been
a problem with titanium and its alloys,
with the most prominent example being
cadmium. Intimate contact (forcing
the titanium into the cadmium or
vice-versa) and high tensile stresses
are required for this to occur.
HIGH COST OF TITANIUM
As many are aware, the primary factor
limiting more extensive use of titanium
is its cost. With a signifi cantly
higher cost than aluminum and steel
alloys, titanium utilization must be
justified for each application. There
are several factors contributing to this.
High energy is required for separation
of the metal from the ore. Ingot
melting is also energy intensive; in
addition its high reactivity requires
melting in an inert atmosphere using
a water-cooled copper retort or hearth,
depending on the melting technique.
Machining is also very high cost, on
the order of 10–100 times slower than
the machining of aluminum alloys. It
was recently pointed out by Froes7 that
a kilogram of aluminum sheet could
be purchased for a lower cost than that of a kilogram of titanium sponge,
the starting material. This sponge still
must be multiple-melted with a master
alloy addition, forged or forged and
rolled to a size appropriate for sheet
bar, put into a pack with multiple sheet
bars, rolled to the appropriated thickness
and etched and ground to the final
thickness to obtain the titanium sheet.
With these factors in mind, much
of the research and development at
Boeing and other original equipment
manufactuers and fabricators is being
devoted to a reduction of the buy-to-fly ratio of titanium components. For
instance, a 40 kg plate may be used
to machine out a 5 kg part, meaning
almost 90% of the titanium is turned
into chips (scrap). Reduction of that
buy-to-fly ratio then means one is
procuring a reduced weight of a very
expensive material, and also reducing
the amount of machining being done
on that material. Several technologies
are being pursued to accomplish this.
These include welding, greater use of
extrusions where appropriate, superplastic
forming and superplastic forming
with diffusion bonding, hot stretch
forming to obtain more precise formed
shapes, and even powder metallurgy.
With regard to welding, both fusion
and solid-state welding are being investigated.
An example of the buy-to-fly reduction which can be achieved
via laser welding is illustrated in Figure
4. Electron beam and friction stir
and linear friction welding are also
being studied. Alloys with improved
machinability are also being pursued.
CONCLUSIONS
Titanium is an attractive material for
numerous industries, but its utilization
has been restricted. A broad range of
activities are underway to reduce this
cost. Significant cost reductions could
greatly expand the industrial base. The
U.S. Army would like to use it for reduced
weight of armored vehicles, the
U.S. Navy would like to use it for the
superstructure of some of its surface
ships as they tend to get top heavy, the
chemical/petrochemical industry could
take greater advantage of its corrosion
resistance, and the aerospace industry
would use more for weight savings if
the price can be driven down. If these
industries could be penetrated in a significant way, the industrial base for
titanium would expand significantly
which should reduce and stabilize the
cost. At present, with the only volume
users being the chemical and aerospace
industries, when the aerospace
industry has a significant pickup in
orders, such as when the Boeing 787
gets up to production rate, the Boeing
requirements will be very high, and
the price will go up. This means that
some of the industries with a positive
but marginal business case may drop
their titanium usage. If the price gets
to the point where the market can be
significantly expanded, the prices
should be more stable.
ACKNOWLEDGEMENTS
The author would like to express
his thanks to Dr. J.C. Williams, Honda
Chair at The Ohio State University
and J.C. Fanning, Manager, Structural
Applications Development at TIMET,
Henderson, Nevada for their helpful
comments and information provided.
REFERENCES
1. R.R. Boyer, Thermec 2003, International Conference
on Processing and Manufacturing of Advanced
Materials (Zurich: Trans Tech Publications, 2003).
2. J.C. Fanning, Titanium 99 Science and Technology
(St. Petersburg, Russia: CRISM, Promety, 2000).
3. M. Burkins et al., “The Mechanical and Ballistic
Properties of an Electron Beam Single Melt of Ti-
6A1-4V Plate,” Army Research Laboratory Report
No. ARL-MR-515 (May 2001), www.arl.army.mil/arlreports/2001/ARL-MR-515.pdf.
4. D.B. Dawson and R.M. Pelloux, Met. Trans., 58 (8)
(1974), p. 723.
5. H. Tobe et al., Ti-2007 Science and Technology (Sendai, Japan: JIM, 2007), p. 1449.
6. F.R. Larson, “Texture in Titanium Sheet and Its
Effect on Plastic Flow Properties,” AMRA TR-65-24 (Alexandria, VA: National Technical Information
Service, 1965).
7. F.H. Froes and M.A. Imam, “Cost Affordable
Developments in Titanium Technology and
Applications,” Cost Affordable Titanium III, ed. M.A.
Imam, F.H. Froes, and K.F. Dring (Zurich: Trans Tech
Publications, 2010), pp. 1–12.
R.R. Boyer is a technical fellow with The Boeing
Company, Seattle, WA 98124; rodney.r.boyer@boeing.com.
|