 | http://www.tms.org/pubs/journals/JOM/0001/Zhitomirsky/Zhitomirsky-0001.html
|
 | http://www.tms.org/pubs/journals/JOM/0001/Zhitomirsky/Zhitomirsky-0001.html
|
Functional Coatings: Overview
 |
|---|
TABLE OF CONTENTS |
Electrodeposition is evolving as an important method in ceramic processing. Two processes for forming ceramic films by cathodic electrodeposition are electrophoretic deposition, in which suspensions of ceramic particles are used, and electrolytic deposition, which is based on the use of metal salts solutions. Electrolytic deposition enables the formation of thin ceramic films and nanostructured powders; electrophoretic deposition is an important tool in preparing thick ceramic films and body shaping.
Electrophoresis was discovered in 1809 by Reuss of Moscow University.
Many processes based on electrophoretic deposition have been described,1,2
including deposition of thick films, laminates, and body shaping. Some of these
processes are in commercial use. Significant interest has recently focused on
cathodic electrodeposition, which offers important advantages for various applications;3
cathodic electrolytic deposition is a new technique in ceramic processing4
that has been used to produce a variety of ceramic thin films.3-22
Electrodeposition offers rigid control of film thickness, uniformity, and deposition
rate and is especially attractive owing to its low equipment cost and starting
materials. Due to the use of an electric field, electrodeposition is particularly
suited for the formation of uniform films on substrates of complicated shape,
impregnation of porous substrates, and deposition on selected areas of the substrates.
Two electrodeposition processes have been developed for forming ceramic films:
electrophoretic deposition (EPD)1-3
and electrolytic deposition (ELD) (Figure
1).3,4
Features of the two processes are shown in Table I.
|
Table I. Electrophoretic and Electrolytic Deposition of Ceramic Materials |
||
|
Electrophoretic
Deposition
|
Electrolytic Deposition
|
|
| Medium | Suspension | Solution |
| Moving Species | Particles | Ions or complexes |
| Electrode Reactions | None | Electrogeneration of OH- and neutralization of cationic species |
| Preferred Liquid | Organic solvent | Mixed solvent (water-organic) |
| Required Conductivity of Liquid | Low | High |
| Deposition Rate | 1-103 mm/min | 10-3-1 mm/min |
| Deposit Thickness* | 1-103 mm | 10-3-10 mm |
| Deposit Uniformity | Limited by size of particles | On nm scale |
| Deposit Stoichiometry | Controlled by stoichiometry of powders used for deposition | Can be controlled by use of precursors |
|
|
||
| *Controlled by variation of
deposition time, voltage, or current density. Controlled by electric field. |
||
 |
|
Figure 1. A schematic of electrolytic deposition and electrophoretic deposition. |
Electrophoretic deposition, a process in which ceramic particles,
suspended in a liquid medium, migrate in an electric field and deposit on an
electrode, has been the subject of considerable interest; review papers are
now available.1,2
Electrophoretic deposition offers important advantages in the deposition of
complex compounds and ceramic laminates. The degree of stoichiometry in the
electrophoretic deposit is controlled by the degree of stoichiometry in the
powder used. According to Reference 1, particle/electrode
reactions are not involved in EPD, and ceramic particles do not lose their charge
on being deposited. The reversal of the electric field results in stripping-off
the deposited layer. Therefore, it is important to use similarly charged particles
and similar solvent-binder-dispersant systems for forming laminates of various
ceramic materials and gaining better control of layer thickness.
A suspension for EPD is a complex system in
which each component has a substantial effect on deposition efficiency. There
are two principal types of solvents used: water and organic liquids. Organic liquids
are superior to water as a suspension medium since the use of water-based suspensions
causes gas formation from the hydrolysis of water. In general, suspensions can
be dispersed by electrostatic, steric, or electrosteric stabilization mechanisms.
Ceramic particles must be electrically charged to permit forming by electrophoretic
deposition. The charge on a colloidal particle could originate from various sources,
such as from adsorbed simple inorganic ions or from dispersants. A binder is also
added to the liquid to increase the adherence and strength of the deposited material
and prevent cracking.
When testing a new ceramic material in the laboratory, polyvinyl butyral as
a binder, phosphate ester as a dispersant, and ethyl alcohol as a solvent were
generally used. Experimental results presented in Reference
23 indicate that phosphate ester is one of the most effective commercial
dispersants, acting as a steric dispersant by anchoring the long chain molecules
to the particle surfaces. Moreover, phosphate ester is an effective electrostatic
stabilizer, which charges the particles positively in organic liquids by donating
protons to the surface.23,24
|
Table II. The
Compositions of Suspensions (SP) and Solutions (SL) and Experimental Conditions
for Constant-Current EPD and ELD
|
|||||
|
Suspension or
Solution
|
Material
|
Additives
|
Solvent
|
Temperature
(° C)
|
Current density
(mA/cm2)
|
|
|
|||||
|
SP1
|
100 g/l TiO2A
|
2.2 g/l PVBG
+ 2.5 g/l PEH
|
Ethyl alcohol
|
20
|
0.1
|
|
SP2
|
100 g/l YSZB
|
3 g/l PVBG
+ 3.5 g/l PEH
|
Ethyl alcohol
|
20
|
0.3
|
|
SP3
|
100 g/l Al2O3C
|
2.3 g/l PVBG
+ 2.7 g/l PEH
|
Ethyl alcohol
|
20
|
0.2
|
|
SL1
|
5 mM TiCl4D
|
0.01 M H2O2I
|
Methyl alcohol-water
(3:1 volume ratio)
|
1
|
20
|
|
SL2
|
5 mM ZrOCl2E
|
-
|
water
|
20
|
20
|
|
SL3
|
5 mM Al(NO3)3F
|
-
|
Ethyl alcohol-water (19:1
volume ratio)
|
20
|
5
|
|
SL4
|
2.5 mM TiCl4D
+ 2.5 mM ZrOCl2E
|
0.02 M H2O2I
|
Methyl alcohol-water
(3:1 volume ratio)
|
1
|
20
|
|
SL5
|
0.02M SnCl4F
|
0.15 M H2O2I
|
Ethyl alcohol-water (19:1
volume ratio)
|
20
|
10
|
|
A Cerac (-325 mesh) B yttrium-stabilized zirconia (YSZ) ,TZ-8Y, Tosoh C Venton, Alfa Division (-400 mesh) D Merck E Fluka Chemie AG F Aldrich Chemical Company G polyvinyl butyral, average Mw = 50,000-80,000, Aldrich Chemical Company H phosphate ester, Emphos PS-21A, Witco I 30 wt.% in water, Carlo Erba Reagenti |
|||||
|
Figure 2. Deposit weight versus time for (a-top) electrophoretic deposits obtained from suspensions SP1-SP3 and (b-bottom) electrolytic deposits obtained from solutions SL1-SL3 at constant current regimes. |
Suspensions for EPD are produced by breaking down agglomerates and uniformly distributing a dispersing agent on the surfaces of the ceramic particles. The particle deagglomeration is carried out by milling and ultrasonic treatment. The preparation of suspensions is carried out in two stages. The dispersant must be added before the binder to prevent competitive adsorption. Figure 2a shows deposit weight versus time dependencies for titania, zirconia, and alumina deposits obtained from suspensions SP1, SP2, and SP3, respectively (Table II). It is seen that deposit weight increases with time at a constant current density. The experimental data presented in Figure 2a demonstrate a manner in which the amount of deposited material can be controlled.
Experiments indicate that the ethyl alcohol-phosphate ester-polyvinyl
butyral system is an effective system for cathodic deposition of various ceramic
materials. This is especially important for deposition of consecutive ceramic
layers of controlled thickness in multilayer processing. Problems related to
the application of toxic solvents, the chemical compatibility of powders and
additives, and deposit contamination and corrosion of electrodes could be eliminated
or diminished. Prepared suspensions exhibited high stability, and a relatively
high deposition rate could be achieved. Due to the use of an effective binder,
obtained deposits adhered well to the substrates and exhibited enhanced stability
against cracking.
The deposition rate depends on applied electric field, suspension concentration,
and electrophoretic mobility of articles.1,2,25-30
When considering other possible factors that can influence the deposition yield,
it is important to note that a certain potential distribution needs to be achieved
in the electrophoretic cell in order to supply sufficient voltage at the electrode
interface and obtain high deposition rates.26
Such potential distribution can be realized by adding an appropriate amount
of phosphate ester or electrolyte. It was shown31-33
that uniformity and adhesion of the deposits can be improved by the use of electrolytes.
However, an increase in the electrolyte concentration caused significant aggregation
of ceramic particles and their sedimentation.31
Particle sedimentation resulted in decreased suspension concentration and was
accompanied by a decrease in the deposition rate.25,31
The deposition process resulted in porous deposits that included a significant
amount of agglomerates.31
It is in this regard that the DLVO theory34,35
explains the existence of a critical electrolyte concentration (flocculation
value) for coagulation, below which the suspension is stable and above which
it is kinetically unstable. The flocculation value decreases with the valence
of the electrolyte ions of a charge opposite to that of the colloidal particles
(rule of Schulze and Hardey).
|
Figure 3. SEM micrographs of (a-top) hollow alumina fiber obtained via EPD and sintered at 1,400°C and (b-bottom) green zirconia deposit obtained via ELD on carbon fiber felt ( photo courtesy of Technimat, Lydall Technical Papers). |
Constant-current or constant-voltage regimes could be used for
EPD. The electric field drives ceramic particles toward the electrode and exerts
a pressure on the deposited layer. It is desirable to maintain a high potential
difference between the anode and the cathode. The use of high voltages has the
advantage of smaller deposition times and higher deposit thickness. It should
be noted that in the case of relatively large particles (~1 mm)
stirring the suspension is usually performed to prevent settling. In this respect,
higher voltages and smaller deposition times are preferable, because shorter
deposition times allow deposition without stirring. It was demonstrated that
electrophoretic phenomena have distinctive features for relatively large particles
(several micrometers) and for particles on a submicrometer scale.25
A high electric field and stirring can induce aggregation and sedimentation
of submicrometer particles, detracting from the deposition process efficiency.
It should be noted that high electric fields bring about porosity in the deposits.25
The use of the electrophoretic process for the deposition of ceramic materials enables the deposition of uniform coatings on substrates of complex shapes. Figure 3a shows hollow alumina fiber obtained via the EPD of submicrometer alumina particles (Baikalox SM-8, Baikowski Ceramic Aluminas) on a carbon fiber and sintering in air at 1,400°C. The obtained deposit was uniform in diameter along the entire fiber length (5 cm). The uniform deposition results from the insulating properties of the deposit and electric field dependence of the deposition rate.3,27,28 However, deposit uniformity is limited by the particle size of the powders used for the deposition process.3,27-29 The possibility to form multilayer structures with controlled layer thickness and sharp interfaces between the layers has been demonstrated.30 Such composites are attracting considerable interest due to their advanced mechanical properties.1 In multilayer fibers obtained via EPD, crack propagation can be deflected at the laminate interfaces.27
Electrolytic deposition produces ceramic materials and provides their deposition. In the cathodic electrodeposition method,4 the following reactions are used to generate base at an electrode surface:
|
2H2O + 2e <==> H2 + 2OH | (1) |
|
NO3
+ H2O + 2e
<==> NO2
+2OH
| (2) |
|
O2 + 2H2O
+ 4e <==> 4OH
| (3) |
Some other cathodic reactions available for the generation of
base have been discussed in the literature.4
Reactions 1-3 consume H2O, generate OH,
and increase the pH at the electrode.
|
Figure 4. The (a-top) electrolytic deposition of ceramic particles and (b-bottom) intercalation of cationic polyelectrolytes into electrolytic deposits. |
In cathodic ELD, metal ions or complexes are hydrolyzed by electrogenerated base (Figure 4a) to form oxide,4-6 hydroxide,7-10 or peroxide11-15 deposits on cathodic substrates. Hydroxide and peroxide deposits can be converted to corresponding oxides by thermal treatment. Hydrolysis reactions result in the accumulation of colloidal particles near the electrode. Turning again to the DLVO theory of colloidal stability,34,35 it may be concluded that the formation of a deposit is caused by flocculation introduced by the electrolyte. The coagulation of colloidal particles near the cathode can be enhanced by the electric field,25 electrohydrodynamic flows,36,37 and pressure resulting from the formation of new particles.
Cathodic ELD is governed by Faraday's law. The amount of the deposited material can be controlled by varying deposition time or current density. Figure 2b shows deposit weight versus time dependencies for titania, zirconia, and alumina deposits obtained from solutions SL1, SL2, and SL3, respectively (Table II). Turning to the data on the EPD of the same materials (Figure 2a), it is seen that the deposition rate in EPD is much faster (by about 1-2 orders of magnitude) than that in ELD (Figure 2b), resulting in higher deposit thickness (Table I).|
Figure 5. X-ray diffraction patterns of deposits obtained from solutions (a) SL1, (b) SL2, (c) SL4, and (d) SL5 and thermally treated at 400°C (SL1, SL2, and SL5) and 700°C (SL4) for 1 h. (O--TiO2, 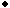 --ZrO2, --ZrO2,
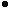 --ZrTiO4,
D--SnO2). --ZrTiO4,
D--SnO2).
|
|
Figure 6. Crystallite sizes of electrolytic titania (anatase) deposits (solution SL1) determined from x-ray data at different temperatures. |
|
Figure 7. The deposit weight of alumina versus cetyltrimethylammonium bromide concentration, 0.1 M Al(NO3)3 solution in ethyl alcohol, deposition time 20 min., current density 5 mA/cm2. |
| M - OH + OH <==> M - O + H2O | (4) |
|
APPLICATIONS |
||
|
There is a growing interest
in electrodeposition of various ceramic materials.1-22,38-59
Electrodeposition has been used for the preparation of thin (ELD4,6,16,40,42)
and thick (EPD1,2,38,39,41,43,44)
films of ferroelectric,16,38
piezoelectric,6,39
magnetic materials,40,41
superconductors,42,43
and semiconductors.4,44
The interest in EPD3,25,28
and ELD45,46
for biomedical applications stems from a variety of reasons, such as
the possibility of deposition of stoichiometric, high-purity material
to a degree not easily achievable by other processing techniques and
the possibility of forming coatings and bodies of complex
shape.3,28 EPD1-3,47-49 and ELD3,4,21,22,50 are especially attractive for the design of solid-oxide fuel cells,21,22,47 solar cells,48 electrochromic devices,49,50 microelectronic devices,1,2,4 fiber-reinforced composites,1,3,4 and batteries.1,4 Protective coatings and electrode materials were deposited via EPD1,2,51,52 and ELD.4,7,9,10,19,20,22 Electrolytic TiO2, RuO2, SnO2, Nb2O5, and composite films4,7,12,13,15,19,20 are of considerable interest for fabrication of dimensionally stable anodes, supercapacitors, and for other electrochemical and catalytic applications.4 Substantial interest in EPD38,43,53 and ELD54,55 has evolved for the deposition of oriented and patterned films. One of the important capabilities provided by EPD56 and ELD57 is the ability to achieve particle impregnation into a porous substrate and composite consolidation. EPD has been demonstrated as a suitable technique for the fabrication of laminar ceramic composites,27,30 functionally gradiented composites,58 hollow fibers and coated fibers,3 phosphor screens,59 and shaping of ceramic bodies.1,2 Electrolytic deposition can be considered as an important tool in the formation of nanostructured materials.4,8,12,17 Other applications of electrophoretic and electrolytic films are discussed in References 1, 2, and 4. |
||
|
|
References
1. P. Sarkar and P.S. Nicholson, "Electrophoretic Deposition
(EPD): Mechanisms, Kinetics, and Applications to Ceramics," J.
Am. Ceram. Soc., 79 (1996), pp. 1987-2002.
2. M.S.J. Gani, "Electrophoretic Deposition--A Review,"
Industrial Ceramics, 14 (1994), pp. 163-174.
3. I. Zhitomirsky, "Electrophoretic and Electrolytic Deposition
of Ceramic Coatings on Carbon Fibers," J.
Europ. Ceram. Soc., 18 (1998), pp. 849-856.
4. I. Zhitomirsky and L. Gal-Or, "Electrochemical Coatings,"
Intermetallic and Ceramic Coatings, ed. Narenda B. Dahotre and T.S. Sudarshan
(New York: Marcel Dekker,
1999), pp. 83-145.
5. I. Zhitomirsky et al., "Electrochemical Preparation of
PbO Films," J. Mater. Sci. Lett., 14 (1995), pp. 807-810.
6. S. Peulon and D. Lincot, "Mechanistic Study of Cathodic
Electrodeposition of Zinc Oxide and Zinc Hydroxychloride Films from Oxygenated
Aqueous Zinc Chloride Solutions," J.
Electrochem. Soc., 145 (1998), pp. 864-874.
7. I. Zhitomirsky and L. Gal-Or, "Ruthenium Oxide Deposits
Prepared by Cathodic Electrosynthesis," Materials Letters, 31 (1997),
pp. 155-159.
8. I. Zhitomirsky and L. Gal-Or, "Characterization of Zirconium,
Lanthanum and Lead Oxide Deposits Prepared by Cathodic Electrosynthesis,"
J. Mater. Sci., 33 (1998), pp. 699-705.
9. R. Chaim et al., "Electrochemical Al2O3-ZrO2
Composite Coatings on Non-Oxide Ceramic Substrates," J. Mater. Sci.,
32 (1997), pp. 389-400.
10. I. Zhitomirsky et al., "Electrochemical Al2O3-Cr2O3
Alloy Coatings on Non-Oxide Ceramic Substrates," J. Mater. Sci., 32 (1997),
pp. 5205-5213.
11. I. Zhitomirsky et al., "Electrodeposition of Ceramic
Films from Non-Aqueous and Mixed Solutions," J. Mater. Sci., 30 (1995),
pp. 5307-5312.
12. I. Zhitomirsky, "Cathodic Electrosynthesis of Titania
Films and Powders," NanoStructured Materials, 8 (1997), pp. 521-528.
13. I. Zhitomirsky, "Electrolytic Deposition of Niobium
Oxide Films," Mater. Letters, 35 (1998), pp. 188-193.
14. I. Zhitomirsky, L. Gal-Or, and S. Klein, "Electrolytic
Deposition of ZrTiO4 Films," J. Mater.
Sci. Lett., 14 (1995), pp. 60-62.
15. I. Zhitomirsky, "Electrolytic Deposition of Oxide Films
in Presence of Hydrogen Peroxide," J.
Europ. Ceram. Soc.,19 (1999), pp. 2581-2587.
16. I. Zhitomirsky, A. Kohn, and L. Gal-Or, "Cathodic Electrosynthesis
of PZT Films," Mater. Lett., 25 (1995), pp. 223-227.
17. I. Zhitomirsky and L. Gal-Or, "Cathodic Electrosynthesis
of Ceramic Deposits," J.
Europ. Ceram. Soc., 16 (1996), pp. 819-824.
18. I. Zhitomirsky et al., "Electrolytic PZT Films," J.
Mater. Sci., 32 (1997), pp. 803-807.
19. I. Zhitomirsky, "Electrolytic TiO2-RuO2
Deposits," J. Mat. Sci., 34 (1999), pp. 2441-2447.
20. I. Zhitomirsky, "Cathodic Electrosynthesis of Titanium
and Ruthenium Oxides," Mater. Lett., 33 (1998), pp. 305-310.
21. H. Konno et al., "Electrochemical Formation of A-Site
Substituted Perovskite La1-xMxCrO3
Oxide Coatings," Electrochimica
Acta, 37 (1992), pp. 2421-2426.
22. H. Konno, M. Tokita, and R. Furuichi, "Formation of
Perovskite Structure La1-xCaxCrO3
Films with Electrodeposition," J.
Electrochem. Soc., 137 (1990), pp. 361-362.
23. K. Mikeska and W. R. Cannon, "Dispersants for Tape Casting
Pure Barium Titanate," Advances in Ceramics--Forming of Ceramics, ed.
J.A. Mangels and G.L. Messing (Columbus, OH: American
Ceramic Society, 1984), pp. 164-183.
24. R. Moreno, "The Role of Slip Additives in Tape-Casting
Technology: Part I-Solvents and Dispersants," Am.
Ceram. Soc. Bull., 71 (1992), pp. 1521-1531.
25. I. Zhitomirsky and L. Gal-Or, "Electrophoretic Deposition
of Hydroxyapatite," J. Mater. Sci., Mater. in Medicine, 8 (1997),
pp. 213-219.
26. J. Mizuguchi, K. Sumi, and T. Muchi, "A Highly Stable
Nonaqueous Suspension for the Electrophoretic Deposition of Powdered Substances,"
J. Electrochem. Soc., 130 (1983), pp. 1819-1825.
27. I. Zhitomirsky and L. Gal-Or, "Formation of Hollow Fibers
by Electrophoretic Deposition," Mater. Lett., 38 (1999), pp. 10-17.
28. I. Zhitomirsky, "Electrophoretic Hydroxyapatite Coatings
and Fibers," Mater. Lett. (in press).
29. I. Zhitomirsky, "Cathodic Electrophoretic Deposition
of Diamond Particles," Mater. Lett., 37 (1998), pp. 72-78.
30. P.S. Nicholson, P. Sarkar, and X. Haung, "Electrophoretic
Deposition and Its Use to Synthesize ZrO2/Al2O3
Micro-Laminate Ceramic/Ceramic Composites," J. Mater. Sci., 28 (1993),
pp. 6274-6278.
31. I. Zhitomirsky, "Electrophoretic Deposition of Chemically
Bonded Ceramics in the System CaO-SiO2-P2O5,"
J. Mater. Sci. Lett., 17 (1998), pp. 2101-2104.
32. M. Shimbo et al., "Electrophoretic Deposition of Glass
Powder for Passivation of High Voltage Transistors," J.
Electrochem. Soc., 132 (1985), pp. 393-398.
33. B.E. Russ and J.B. Talbot, "An Analysis of the Binder
Formation in Electrophoretic Deposition," J.
Electrochem. Soc., 145 (1998), pp. 1253-1256.
34. B.V. Derjaguin and L. Landau, "Theory of Stability of
Highly Charged Lyophobic Sols and Adhesion of Highly Charged Particles in Solutions
of Electrolytes," Acta Physicochim. USSR, 14 (1941). pp. 633-652.
35. E.J.W. Verwey and J.Th.G. Overbeek, Theory of Stability
of Lyophobic Colloid (Amsterdam, Netherlands: Elsevier,
1948).
36. Y. Solomentsev, M. Bφhmer, and J.L. Anderson, "Particle
Clustering and Pattern Formation during Electrophoretic Deposition: A Hydrodynamic
Model," Langmuir, 13 (1997), pp. 6058-6068.
37. M. Trau, D.A. Saville, and I.A. Aksay, "Assembly of
Colloidal Crystals at Electrode Interfaces," Langmuir, 13 (1997), pp.
6375-6381.
38. M. Okutomi et al., "Evolution of Microstructure in BaTiO3
Thin Films Recrystallized by Laser," Surface Engineering, 13 (1997),
pp. 66-70.
39. S. Sugiyama, A. Takagi, and K. Tsuzuki, "(Pb,La)(Zr,Ti)O3
Films by Multiple Electrophoretic Deposition/Sintering Processing," Jpn.
J. Appl. Phys., 30 (1991), pp. 2170-2173.
40. G. Zotti et al., "Electrodeposition of Amorphous Fe2O3
Films by Reduction of Iron Perchlorate in Acetonitrile," J.
Electrochem. Soc., 145 (1998), pp. 385-389.
41. N. Koura et al., "Preparation of Various Oxide Films
by an Electrophoretic Deposition Method: A Study of the Mechanism," Jpn.
J. Appl. Phys., 34 (1995), pp. 1643-1647.
42. S.B. Abolmaali and J.B. Talbot, "Synthesis of Superconductive
Thin Films of YBa2Cu3O7-x
by a Nonaqueous Electrodeposition Process," J.
Electrochem. Soc., 140 (1993), pp. 443-445.
43. P. Sarkar et al., "Fabrication of Textured Bi-Sr-Ca-Cu-O
Thick Film by Electrophoretic Deposition," J.
Appl. Phys., 69 (1991), pp. 1775-1777.
44. F. Lindner and A. Feltz, "Thin Layer NTC Semiconductor
Ceramics Based on NiMn2O4
and ZnzNiMn2-zO4
(z=1/3,2/3)," J.
Europ. Ceram. Soc., 11 (1993), pp. 269-274.
45. M. Shirkhanzadeh, "Direct Formation of Nanophase Hydroxyapatite
on Cathodically Polarized Electrodes," J. Mater. Sci.: Mater. in Medicine,
9 (1998), pp. 67-72.
46. S. Ban and S. Maruno, "Deposition of Calcium Phosphate
on Titanium by Electrochemical Process in Simulated Body Fluid,"
Jpn. J. Appl. Phys., 32 (1993), pp. L1577-L1580.
47. T. Ishihara, K. Sato, and Y. Takita, "Electrophoretic
Deposition of Y2O3-Stabilized
ZrO2 Electrolyte Films in Solid Oxide Fuel
Cells," J.
Am. Ceram. Soc., 79 (1996), pp. 913-919.
48. E.W. Williams et al., "The Electrophoresis of Thin Film
CdS/Cu2S Solar Cells," Solar Cells,
1 (1979/80), pp. 357-366.
49. K. Kuwabara, K. Sugiyama, and M. Ohno, "All-Solid State
Electrochromic Device. 1. Electrophoretic Deposition Film of Proton Conductive
Solid Electrolyte," Solid State Ionics, 44 (1991), pp. 313-318.
50. T. Yoshino and N. Baba, "Characterization and Properties
of Electrochromic Cobalt Oxide Thin Film Prepared by Electrodeposition," Solar
Energy Materials and Solar Cells, 39 (1995), pp. 391-397.
51. C. Song and G. Villemure, "Preparation of Clay-Modified
Electrodes by Electrophoretic Deposition of Clay Films," J. Electroanalytical
Chem., 462 (1999), pp. 143-149.
52. C.B. Ahlers and J.B. Talbot, "Fabrication of Zeolite-Modified
Electrodes via Electrophoretic Deposition," J.
Electrochem. Soc., 146 (1999), pp. 3259-3263.
53. S.W. Kang, J.S. Yoo, and J.D. Lee, "Photolithographic
Patterning of Phosphor Screens by Electrophoretic Deposition for Field Emission
Display Application," J. Vac. Sci. Technol. B., 16 (1998), pp. 2891-2893.
54. K.J. Stevenson, G.J. Hurtt, and J.T. Hupp, "High Resolution
Assembly of Patterned Metal Oxide Thin Films via Microtransfer Molding and Electrochemical
Deposition Techniques," Electrochemical and Solid-State Lett., 2 (1999),
pp. 175-177.
55. M. Izaki and T. Omi, "Characterization of Transparent
Zinc Oxide Films Prepared by Electrochemical Reaction," J.
Electrochem. Soc., 144 (1997), pp. 1949-1952.
56. L. Gal-Or, S. Liubovich, and S. Haber, "Deep Electrophoretic
Penetration and Deposition of Ceramic Particles Inside Porous Substrates II.
Experimental Model," J.
Electrochem. Soc., 139 (1992), pp. 1078-1081.
57. K.-C. Ho and J. Jorne, "Electrochemical Impregnation
of Nickel Hydroxide. Flow-Through vs. Stagnant Electrodes," J.
Electrochem. Soc., 137 (1990), pp. 149-158.
58. P. Sarkar, X. Huang, and P.S. Nicholson, "Zirconia/Alumina
Functionally Gradiented Composites by Electrophoretic Deposition Techniques,"
J.
Am. Ceram. Soc., 76 (1993), pp. 1055-1056.
59. J.A. Siracuse et al., "The Adhesive Agent in Cataphoretically
Coated Phosphor Screens," J.
Electrochem. Soc., 137 (1990), pp. 346-348.
Igor Zhitomirsky is with the Department of Materials
Science and Engineering, McMaster
University.
For more information, contact I. Zhitomirsky, Department of Materials Science
and Engineering, McMaster University, 1280 Main Street West, Hamilton, Ontario,
Canada, L8S 4L7; fax (905) 528-9295; e-mail zhitom@mcmaster.ca.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|